Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Mechanics Of G Protein-Coupled Receptors Unraveled
Molecular Biology: Researchers use computational and experimental techniques to nail down how the receptors activate G protein signaling
by Stu Borman
June 22, 2015
| A version of this story appeared in
Volume 93, Issue 25

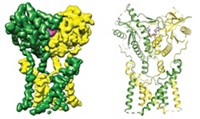
Cell signals produced by G protein-coupled receptors (GPCRs) play a role in many parts of daily life—for example, the sense of smell or taste, food digestion, and learning. Because GPCRs are involved in so many biological events, about one-third of drugs, including antihistamines and psychiatric medications, target GPCRs. And studies of how GPCRs work earned two researchers—Robert J. Lefkowitz and Brian K. Kobilka—the 2012 Nobel Prize in Chemistry.
But scientists had not known the molecular details of how these important receptors activate cell signaling. Now, researchers have used computer simulations and experiments to help answer that question.
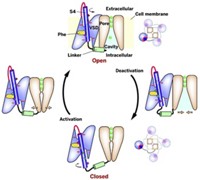
In the signaling process, biomolecules bind to GPCRs on cell surfaces. G proteins inside the cells then bind to the receptors, and a guanosine diphosphate (GDP) bound to the G protein gets replaced with guanosine triphosphate (GTP).
The G protein is known to have a wide-open conformation as the exchange takes place. What hasn’t been known is exactly how the GPCR promotes GDP’s escape. Does the GPCR force a closed G protein to open and release GDP, or does it aid GDP release in a G protein that has opened by itself?
Now, experimentalists who carried out earlier studies on the activation process have teamed up with computationalists Ron O. Dror, currently at Stanford University, and David E. Shaw and coworkers at D. E. Shaw Research, in New York City, to address that question (Science 2015, DOI: 10.1126/science.aaa5264).
The computationalists performed atomic-level molecular dynamics simulations of G proteins alone or bound to a GPCR. The experimentalists then used double electron-electron resonance spectroscopy, protein engineering, and other techniques to confirm the results of the simulations.
The combined studies revealed that the GPCR does not force open the G protein. Instead, when a G protein binds the receptor, it is already open or opens later on its own. The GPCR rearranges the conformational furniture inside the open G protein, promoting GDP release. GTP then binds to replace the missing GDP.
“This is a lovely piece of work that integrates multiple and highly sophisticated approaches,” comments GPCR expert Henrik G. Dohlman of the University of North Carolina School of Medicine. The simulations were able to help answer this important question because they were orders of magnitude longer than previous ones, he says. “It helps that Shaw Research has a really kick-ass supercomputer.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter