Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Chemists Demonstrate New Feats Of Hydroamination
Organic Synthesis: Two groups turn underutilized internal alkenes and alkynes into chiral amines
by Stephen K. Ritter
July 2, 2015
| A version of this story appeared in
Volume 93, Issue 27

In a showcase of metal hydride catalysis, two research groups have developed reactions to transform simple unsaturated building blocks—alkenes and alkynes—into valuable chiral amine products.
What makes these so-called hydroamination reactions noteworthy is that the multiple bonds are located at internal carbon atoms in the molecules rather than at terminal positions. Organic chemists have long considered it a challenge to easily transform the less reactive internal unsaturated bonds.
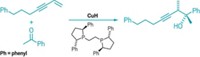
In the case of alkenes, a team led by Stephen L. Buchwald of Massachusetts Institute of Technology solved the challenge with the help of a chiral copper hydride catalyst incorporating an aromatic bidentate phosphine ligand (Science 2015, DOI: 10.1126/science.aab3753).
In the past, metal hydrides have had a low binding affinity for internal alkenes and the resulting intermediates have reacted sluggishly with nitrogen nucleophiles such as secondary amines. Buchwald’s group flipped that approach around and created a copper catalyst that forms a nucleophilic alkylcopper intermediate, which allows the team to use an electrophilic nitrogen oxidant—a hydroxylamine ester—as the amine source. As a result, chemists can now take a basic building block such as 2-butene and turn it into a range of enantiopure amines.
In the case of alkynes, Vy M. Dong and coworkers at the University of California, Irvine, have developed the first example of an enantioselective alkyne hydroamination (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b05200). The key was exploiting the ability of their catalyst, a rhodium hydride paired with a chiral bidentate aliphatic phosphine ligand, to isomerize alkynes to form an electrophilic rhodium-allyl complex. When treated with a secondary amine, the complex undergoes hydroamination to generate allylic amines.
The researchers found that the choice of an organic acid additive—m-xylylic acid versus phthalic acid—helps guide the hydroamination to selectively form chiral branched amines or linear amines, respectively. All previous alkyne hydroaminations have yielded only achiral enamines, imines, or linear allylic amines.
Between the two transformations, both research groups agree the internal alkene hydroamination ranks higher in importance. “The demonstration of enantioselective copper-catalyzed alkene hydroamination addresses one of the long-standing challenges in catalysis, namely the selective synthesis of nitrogen-carbon bonds from abundant alkene precursors and amines,” says Paul J. Chirik, a chemistry professor at Princeton University and editor-in-chief of the journal Organometallics. “The Buchwald work also demonstrates the value of integrating theory and experiment to solve important problems in synthetic methodology.”
The new approaches should inspire further advances on the emerging theme of using hydrofunctionalization for preparing complex molecules, Dong says. For example, hydroformylation (the oxo process) to convert an olefin into an aldehyde is already the leading example of homogeneous catalysis in the chemical industry, she notes. Other metal-catalyzed hydrofuctionalizations, such as hydroamination, could follow suit. “They have enormous potential,” Dong says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter