Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Tomography Maps Aluminum Clusters In Zeolites
Materials: Technique could help improve performance of the industrially critical catalysts
by Mitch Jacoby
July 13, 2015
| A version of this story appeared in
Volume 93, Issue 28

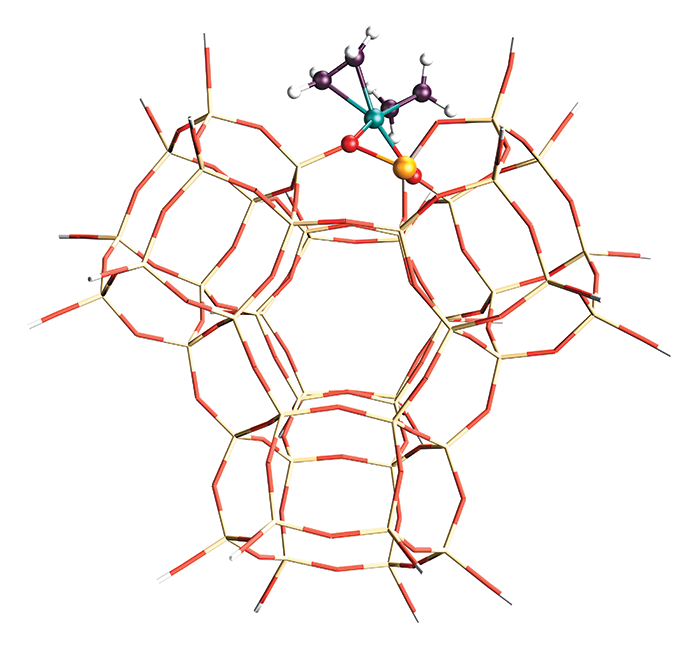
Zeolites catalyze the petroleum refining process that produces the majority of the world’s gasoline. The catalytic prowess of these porous solids emanates from aluminum atoms sprinkled throughout their silicate frames. Until now, researchers have struggled to determine precisely where the aluminum atoms reside.
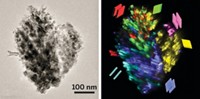
By using atom probe tomography (APT), a relatively uncommon technique that maps elements in three dimensions, an international team of researchers has pinpointed the locations of aluminum atoms in a commercial zeolite known as ZSM-5 (Nat. Commun. 2015, DOI: 10.1038/ncomms8589). The group has also learned how steaming, a standard procedure for activating the catalysts, affects aluminum’s distribution in the solids. The study may lead to methods for making better-performing, longer-lasting catalysts.
In a typical zeolite structure, AlO4 units scattered among SiO4 units introduce negative charges that are typically balanced by protons. These positive charges form Brønsted acid sites that are responsible for much of zeolites’ knack for cracking large molecules in crude oil, producing smaller, more valuable compounds.
Earlier studies suggested that aluminum is distributed nonuniformly in zeolite crystals. But because of the small mass difference between aluminum and silicon, studies based on X-ray diffraction, electron microscopy, and other techniques have come up short in identifying where aluminum sits.
To get the answer, Bert M. Weckhuysen of Utrecht University in the Netherlands and Simon R. Bare of UOP, a refining technology company in Des Plaines, Ill., teamed up with APT experts Daniel E. Perea and Ilke Arslan of Pacific Northwest National Laboratory and others.
The group mapped the 3-D distribution of individual aluminum atoms and determined distances among neighboring aluminums, confirming that the element is not distributed randomly in ZSM-5 crystals. They showed that steam-treating the zeolite leads to aluminum segregation and clustering, especially along crystal defects known as grain boundaries.
This use of APT provides “a major advance in spatial mapping of light elements in zeolites,” by providing scientists with structural information unavailable until now, says University of Delaware chemical engineering professor Raul F. Lobo, who is a zeolite specialist.
Lobo explains that Brønsted acid sites that are found throughout zeolites can exhibit vast differences in catalytic properties because of the influence of nearby atoms. Crystallography provides structural information averaged over large numbers of sites, he says. “Yet, the actual bond-breaking and bond-making processes happen on specific, not average, sites.” Noting the method may be applied to many catalysis problems, Lobo adds, “The APT technique described here gets us much closer to the specific and detailed information we are looking for.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter