Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists Iron Out [2+2] Cycloadditions
Organic Synthesis: Tunable catalyst simplifies turning commodity alkenes into cyclobutane building blocks
by Stephen K. Ritter
August 27, 2015
| A version of this story appeared in
Volume 93, Issue 34
A team of Princeton University researchers has tinkered with the ligand design of an iron catalyst to overcome a long-standing reactivity barrier for coupling alkenes to make cyclobutanes. The developments by Jordan M. Hoyt, Paul J. Chirik, and coworkers provide chemists new options for converting abundant olefin feedstocks such as propylene and 1-octene into diverse sets of chemical building blocks.
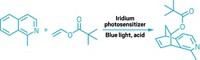
The Princeton team focused on [2+2] cycloaddition reactions that weld together a pair of alkenes or an alkene and a diene to make cyclobutanes. [4+2] Cycloadditions, which are used to make six-membered rings, proceed readily under mild reaction conditions. In contrast, [2+2] cycloadditions require an extra kick to overcome the electron orbital restraints of producing a smaller four-membered ring.
To give the reaction a nudge, chemists often use light to stimulate at least one of the coupling partners, but simple alkenes are not amenable to this photoactivation. Metal catalysts have also been used, but poor substrate selectivity typically leads to mixtures of products and yields remaining too low to be useful.
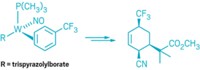
Chirik’s group previously reported an iron pyridine(diimine) catalyst that facilitates [2+2] cycloadditions of unactivated dienes to prepare bicyclic compounds. With further work, they have now discovered how subtle changes in the alkyl and aryl substituents around the edges of the iron complex help control the oxidation state and steric environment of the iron center to influence catalyst activity toward simple alkenes (Science 2015, DOI: 10.1126/science.aac7440).
One of their new catalysts bearing bulky ligands promotes cyclodimerization of propylene or terminal alkenes to form various cyclobutanes. When the catalyst is outfitted with smaller ligand groups, it promotes tail-to-tail alkene dimerizations, for example, converting two propylene molecules into 2,3-dimethylbutene. The team introduced other alterations in the ligand system to achieve alkene-diene cross-cycloadditions, leading to more diverse cyclobutanes.
The Princeton method “brings unprecedented scope to this ostensibly simple yet deceptively challenging transformation,” write Myles W. Smith and Phil S. Baran of Scripps Research Institute California in an accompanying perspective article. “This compelling discovery will no doubt spur development of more robust and versatile catalyst platforms for [2+2] cycloadditions of commodity olefins and beyond.”

![Reaction scheme showing an iron-catalyzed [2+2] cycloaddition. Reaction scheme showing an iron-catalyzed [2+2] cycloaddition.](https://s7d1.scene7.com/is/image/CENODS/09334-notw3-scheme-500?$responsive$&wid=300&qlt=90,0&resMode=sharp2)


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter