Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Simple Method Crosslinks And Improves Perovskite Crystals
Photovoltaics: Hydrogen-bonding phosphonic acid compound protects solar cells from heat and moisture
by Mitch Jacoby
August 31, 2015
| A version of this story appeared in
Volume 93, Issue 34
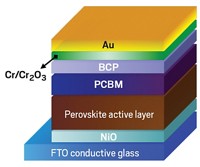
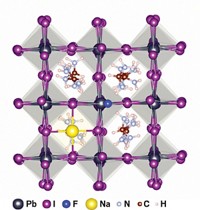
A one-step wet-chemistry procedure cross-links and stabilizes crystals of methylammonium lead triiodide, a perovskite material widely studied for application in photovoltaics (Nat. Chem. 2015, DOI: 10.1038/nchem.2324). The advance may improve the durability of solar cells based on trihalide perovskite compounds and spur these low-cost devices, which convert sunlight to electricity, toward commercialization. The rapid rise in perovskite solar cells’ power conversion efficiency (PCE) has thrust these experimental devices into the photovoltaics limelight. But their tendency to degrade upon exposure to heat and humidity leaves them with an uncertain future. A team led by Michael Grätzel of ETH Lausanne may have a way to stop the degradation. The group reports that spin coating a solution of the perovskite precursors and butylphosphonic acid 4-ammonium chloride forms well-ordered perovskite crystals cross-linked via hydrogen bonds. In tests conducted at 55% relative humidity, the PCE of solar cells treated with the additive slowly fell from 14% to 10% over 40 days. The PCE of untreated cells fell from 7% to 1% in just one week. Other tests showed that unlike untreated cells, treated ones remained fairly stable when exposed to 85 °C temperatures for 350 hours.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter