Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
First Metal-Organic Framework Made With Protein
Bioinorganic Chemistry: Engineered protein, metal ion, and linker form new type of hybrid material
by Stu Borman
September 2, 2015
| A version of this story appeared in
Volume 93, Issue 35

Chemists have engineered a protein so that it self-assembles with zinc ions and an organic small molecule to form the first protein-based metal-organic framework (MOF).
Including proteins in MOFs could combine the biocatalytic, electron-transfer, and molecular recognition capabilities of proteins with the separation, storage, and catalysis applications of MOFs.
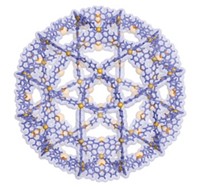
Traditional MOFs, which have attracted great academic and industrial interest in recent years, consist of organic linkers connecting metal centers. Akif Tezcan at the University of California, San Diego, and colleagues are now making MOFs even more versatile by demonstrating that the materials can be synthesized with protein centers (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b07463).
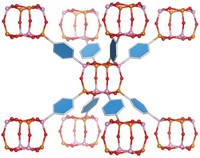
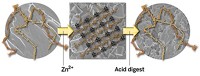
The researchers engineered human ferritin with metal-binding sites, permitting each protein molecule to accommodate eight zinc ions on its surface. When the engineered protein is mixed with zinc and benzenehydroxamic acid, zinc binds to the protein and benzenehydroxamic acid acts as a linker, chelating zinc ions on separate ferritins. This self-assembly process results in a MOF crystal in which each zinc-decorated ferritin is linked to eight neighboring ones.
In addition to creating a new type of MOF, the work “introduces a new strategy for the rational design of protein crystals,” Tezcan says. Self-assembling protein networks have been created before, but earlier structures were made from two independent components instead of three and formed random structures instead of crystalline ones. The addition of a third component adds design versatility to the materials, and crystals are more uniform and well-defined than noncrystalline substances. Chemists have made MOFs with amino acids or short peptides, but never before with a functional protein such as ferritin, which acts in the body to store iron.
“The material couples the ability to design, fine-tune, and crystallize MOFs with the chemical and structural diversity of proteins,” says MOF expert Omar Yaghi of the University of California, Berkeley. “By placing proteins as structural elements into MOFs, one fixes them in position to serve as addressable entities for reactions and controlled dynamics.”
Seong Huh of Hankuk University of Foreign Studies, in Yongin, South Korea, says, “This is a unique approach to immobilize functional proteins into an inorganic matrix.” Huh and Seongsoon Park of Sungshin Women’s University, in Seoul, created an earlier protein-modified MOF. They think the new MOF could be useful for protein drug delivery, new types of biocatalysts, and molecular electronic devices.
But Huh notes that it remains to be seen if other proteins can form crystalline protein-MOFs as readily as ferritin. The metal-binding protein is roughly spherical, making it more amenable to crystallization than less symmetrical proteins.
Manuel A. Navia of Oxford Bioscience Partners, who developed and commercialized cross-linked enzyme crystals, agrees that the ferritin-based MOF “may be a special case and the global exploitation of the protein-MOF concept may be difficult.”
Still Navia thinks this novel extension of MOFs represents an unexplored, new form of matter. “The work should get a lot of people thinking about really cool experiments they could do with these materials,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter