Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Organometallics Add Aryl Groups To Proteins Selectively
Bioconjugation: Palladium reagent works in water to tack small molecules onto cysteine residues
by Bethany Halford
October 29, 2015
| A version of this story appeared in
Volume 93, Issue 43

Biochemists may soon find themselves reaching for palladium reagents—chemicals once thought to be exclusively used in organic synthesis—to couple small molecules, such as drugs, onto proteins. Chemists at MIT have developed a reaction that selectively adds aryl groups to free cysteine residues in proteins. The reaction can be used to make myriad modified proteins, including therapeutic antibody-drug conjugates.
Palladium compounds have long been workhorse reagents for coupling components of small molecules. But they haven’t found much use in similar coupling reactions with proteins because they tend not to work under the particular conditions the biomolecules require: aqueous solution, low temperature, and mild pH.
Now, Bradley L. Pentelute, who specializes in bioconjugation chemistry, and Stephen L. Buchwald, an expert in palladium coupling chemistry, have combined forces to come up with a simple method to modify proteins with palladium reagents (Nature 2015, DOI: 10.1038/nature15739). Other chemists have previously used the metal to modify proteins, but these reactions required unnatural amino acids or the addition of functional groups to natural amino acids.
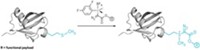
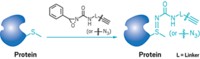
The reaction the Pentelute and Buchwald groups came up with uses an aryl palladium reagent to couple an aryl group selectively onto the sulfur of a cysteine residue. The reaction quantitatively modifies a protein’s free cysteine residues in a matter of minutes at room temperature in aqueous solution with 5% organic cosolvent. The resulting cysteine-modified proteins are quite stable.
The MIT chemists used the reaction to prepare several modified biomolecules, including a degradation-resistant type of peptide called a stapled peptide and a conjugate between an antibody and the anticancer drug vandetanib.
The key to the reaction’s success, says Ekaterina V. Vinogradova, one of the lead authors on the paper, is its speed. The reaction between the palladium and the cysteine’s sulfur happens so quickly that there’s no time for other competing reactions to take place. “We never expected that the reaction would be so fast and robust,” Vinogradova tells C&EN. “We can basically take almost any aryl halide or aryl triflate and put it on a biomolecule.”
“I think it’s a very powerful strategy,” comments Heather Maynard, an expert in protein conjugation chemistry at the University of California, Los Angeles. She expects that biochemists will be more likely to adopt the technique if they don’t have to make the reagents but can buy them in a kit instead. “It’s high yielding, it’s fast, and the fact that it has a relatively stable bond compared to other chemistry makes this reaction very exciting,” she says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter