Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Nickel Shines As A Catalyst
Organic Synthesis: Research advances continued a trend to move away from precious-metal catalysts
by Stephen K. Ritter
December 21, 2015
| A version of this story appeared in
Volume 93, Issue 49

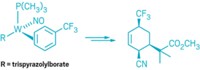
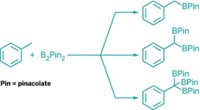
Transition-metal-catalyzed reactions are wildly successful in organic synthesis, in particular for cross-couplings that construct carbon-carbon and carbon-heteroatom bonds. Palladium catalysts have dominated these reactions, but chemists are interested in switching to more abundant, lower-cost metals such as iron, nickel, and other first-row metals. In 2015, nickel continued to shine in this role as chemists coaxed it to carry out a broader array of reactions. In one example, Mark Stradiotto and coworkers of Dalhousie University reported the first case of nickel-catalyzed arylation of ammonia—the simplest and most abundant N–H source in chemistry—to make amines. The researchers used air-stable Ni(cyclooctadiene)2 or NiCl2(dimethoxyethane) with a ferrocenyl phosphine ligand known as JosiPhos to link up substituted aryl and heteroaryl bromides, chlorides, and tosylates with ammonia to make diverse aryl and heteroaryl amines (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201410875). In another example, a team led by Kendall N. Houk and Neil K. Garg at the University of California, Los Angeles, developed a nickel cyclooctadiene/N-heterocyclic carbene catalyst that makes short work of cleaving and modifying normally unreactive amide functional groups (Nature 2015, DOI: 10.1038/nature14615). Garg’s group capitalized on the approach by achieving the first nickel-catalyzed Suzuki-Miyaura reaction using an amide derivative as a cross-coupling partner (Nat. Chem. 2015, DOI: 10.1038/nchem.2388). Researchers have also been discovering methods that couple nickel catalysts with other catalysts to perform new chemistry. In one twist on cross-coupling, Daniel J. Weix and his group at the University of Rochester reported pairing a nickel bipyridine catalyst that reacts with aryl bromides and a palladium phosphine catalyst that reacts with aryl triflates. The surprising result was the first general method for making unsymmetrical biaryl compounds directly from two different aryl electrophiles (Nature 2015, DOI: 10.1038/nature14676). “Using nickel is especially advantageous nowadays,” Chris H. Senanayake, vice president of chemical development at Boehringer Ingelheim Pharmaceuticals, told C&EN. “There is a dramatic shift taking place in industrial process chemistry from palladium to non-precious-metal catalysis.”


C&EN's YEAR IN REVIEW
Top Headlines of 2015
- Chemical Makers Looked To Big Deals
- NASA Got Up Close And Personal With Pluto
- Opposition To Neonicotinoids Intensified
- 2015 Nobel Prizes In Science At A Glance
- Climate Pact Clinched
- Little Good News For Chemistry Job Outlook In 2015
- Pfizer To Merge Again, This Time With Allergan
- Oil And Gas Industry Under Pressure
- Greening Up Fracking
- An Industry In Spin Cycle
- Finally, Emoji For Chemists
- A Big Deal For Chemists
- Tianjin Explosion Put Spotlight On Safety
- Women Assumed Leadership Roles At American Chemical Society
- Artificial Ingredients In The Crosshairs
- Jacqueline K. Barton, Unwavering Chemistry Champion
- House Science Committee Chair Pummeled Science Agencies
- NIST Veteran Became U.S. Government's Top Chemist
- Gene-Editing Technique Raised Ethics Questions
- World Chemical Production At A Glance
- Classroom Fires During Science Demonstrations Spark Concern
- Climate Was Right For Deal-Making
- American Chemical Society Dives Deeper Into Open Access With The Debut Of ACS Omega
- Lego Began Research On Switching To A Biobased Plastic
- New Chair Took The Helm At Troubled Chemical Safety Board
- Genetically Modified Foods In The Spotlight
- Overhaul Of U.S. Chemical Law Moved
- ACS Scholars Program Turned 20
- American Chemical Society Expanded Its Global Reach
- Scientists Called For Standardized Antibodies
Top Research of 2015
- Flexible Electronics You Can Inject
- Special Delivery For Sensitive Reagents
- Yeast Programmed For Opioid Total Synthesis
- Miracle Machine Builds Molecules On Demand
- Nickel Shines As A Catalyst
- 3-D Printing Takes On A New Dimension
- Atomically Thin Films Grow In Number
- Digging In The Dirt Yields Novel Bacteria Fighter
- Keeping GMOs On A Leash
- A Liquid With Holes In It
- Electron Microscopy Provides Unprecedented Close-ups
Revisiting Research of 2005



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter