Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Understanding Tooth Enamel’s Hardness
Researchers discover how the noncrystalline components of enamel help keep the material hard and corrosion resistant
by Sarah Everts
February 16, 2015
| A version of this story appeared in
Volume 93, Issue 7
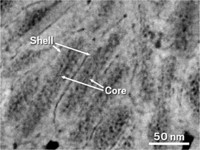
Tooth enamel has a tough job. Surrounded by hostile mouth bacteria that produce corrosive acid, enamel must withstand the wear and tear of grinding food. Scientists have known that well-ordered crystalline hydroxyapatite nanowires help enamel resist cracking and form the bulk of the material. But a new study suggests how amorphous components—present in only small amounts—can deliver significant hardness and corrosion resistance to enamel (Science 2015, DOI: 10.1126/science.1258950). Northwestern University’s Derk Joester and colleagues used a potpourri of physical techniques to peer at the complex structure of pearly rabbit, mouse, and rat enamel as well as stained beaver enamel, which is harder and more resistant to acid attack. They found that the amorphous parts of normal enamel are composed primarily of magnesium-substituted amorphous calcium phosphate (Mg-ACP). Beaver teeth get their added hardness and their stain from the replacement of Mg-ACP with iron-containing mineral and phosphates. This study may eventually lead to enamel-strengthening strategies that prevent or minimize cavities, the authors note.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter