Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Reconstructed Ancestral Proteins Answer Question About Gleevec’s Binding Mechanism
Researchers retrace evolutionary paths of two modern kinases to learn why the well-known cancer drug targets one enzyme but not the other
by Celia Henry Arnaud
February 23, 2015
| A version of this story appeared in
Volume 93, Issue 8
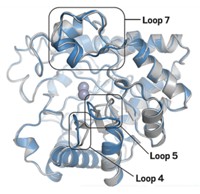
The leukemia drug Gleevec is exquisitely selective. It binds strongly to and inhibits a kinase enzyme known as Abl but doesn’t bind much to the closely related enzyme Src. Scientists have not been able to account for the 3,000-fold difference in binding affinity for the two kinases. To understand Gleevec’s preference, Dorothee Kern and coworkers at Brandeis University reconstructed Abl and Src’s last common ancestral protein and other proteins along the evolutionary paths leading to the modern kinases (Science 2015, DOI: 10.1126/science.aaa1823). The ancestral protein binds Gleevec with an affinity between that of Abl and Src. The affinity increased along the evolutionary trail toward Abl and decreased along the way to Src. The researchers attribute the changing affinities to conformational shifts in a protein loop that holds Gleevec in place. Although modern Abl and Src differ by 146 amino acids, the team’s analysis pointed to just 15 amino acids that are responsible for Gleevec’s selectivity. Most of them are far from the drug’s binding pocket, which highlights the importance of dynamic changes occurring in a protein far from this site.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter