Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Raman Technique Reveals Protein Synthesis In Live Tissue
Biological Imaging: Method could be used to track protein metabolism related to memory formation or disease
by Melissae Fellet
January 15, 2015

A newly improved imaging method helps researchers map where cells are synthesizing and degrading proteins in live tissue (ACS Chem. Biol. 2015, DOI: 10.1021/cb500787b). The technique, based on Raman spectroscopy, could be used to investigate how protein metabolism changes in cognitive disorders or long-term memory formation, the researchers say.
To better understand how our brains learn and remember, neuroscientists want to pinpoint which neurons fire and which synapses are active when the brain encodes new information. Visualizing the location and speed of protein synthesis is one way to identify active parts of the brain. Common techniques to image protein synthesis involve tagging amino acids with radioactive, fluorescent, or isotopic labels. Researchers use microscopy or mass spectrometry to visualize the labeled amino acids that cells incorporate into new proteins.
Those imaging methods, however, provide just a snapshot of protein synthesis, says Wei Min of Columbia University. The techniques require fixing the cells or tissue, so scientists cannot follow how protein synthesis changes over time.
In 2013, Min and his colleagues developed a method to visualize protein synthesis in live cells (Proc. Natl. Acad. Sci. USA 2013, DOI: 10.1073/pnas.1303768110). Now they wanted to use it to track protein synthesis in live tissue.
In this method, the researchers incubate cells with amino acids containing deuterium instead of hydrogen. The cells incorporate the deuterated amino acids into new proteins. Then the scientists detect the labeled amino acids using stimulated Raman scattering microscopy. This technique can pick up the vibration of the carbon-deuterium bond as a unique signal among all the molecules in the cell.
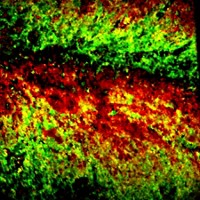
To test the method on live tissue, the researchers cultured a slice from the hippocampus of a mouse brain with deuterated amino acids. The team improved the method’s sensitivity by increasing the amount of deuterated amino acids absorbed by the cells. This also allowed them to speed up the rate at which they captured images. When they used the Raman technique to look at the tissue, the researchers saw areas of protein synthesis in individual neurons in the cortex, as well as in the dentate gyrus, an area associated with memory formation.
Min says this is the first measurement of protein synthesis in live brain tissue.
Through other experiments, the researchers also identified a Raman signal unique to the carbon-hydrogen bonds of methyl groups on proteins synthesized before the researchers introduced their deuterated amino acids. To keep the total number of proteins constant, cells degrade existing proteins as they synthesize new ones. Some disorders that affect cognition, like Huntington’s disease, involve altered protein degradation pathways.
The team used Raman imaging to follow the methyl signal in brain slices and visualize areas where proteins were being degraded. Unlike localized regions of protein synthesis, areas of protein degradation were relatively consistent throughout the brain slice.
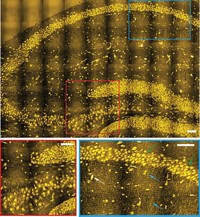
Min and colleagues also imaged protein synthesis in whole organisms like zebrafish. They injected deuterated amino acids into embryonic zebrafish and fixed the embryos. The researchers observed protein synthesis in the fish embryos’ tails.
“To see where new protein is synthesized in a living creature is really significant,” says Eric O. Potma of the University of California, Irvine. This elegant work, he adds, demonstrates the power of using nonlinear Raman spectroscopy for imaging applications.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter