Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Evolving Toward Universal Blood
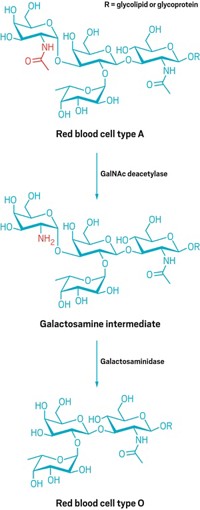
Bioengineering: Researchers improve the efficiency of an enzyme that turns A-type red blood cells into a universal type
by Louisa Dalton
May 8, 2015

Hospitals keep stores of universal, type O blood for situations when a patient with an unknown blood type needs an emergency transfusion. The other types—A, B, and AB blood—can trigger a potentially fatal immune response in an unmatched recipient. Now, bioengineers have taken a step on the path toward making all blood universal—by broadening an enzyme’s ability to remove antigens on the surface of red blood cells (J. Am. Chem. Soc. 2015, DOI: 10.1021/ja5116088).
The quest for universal blood has tempted researchers since the discovery in the 1980s of coffee bean enzymes that could turn type B blood into O. The four main blood types each have distinctive sugar chains on the surface of their red blood cells. The chain on O-type cells consists of four sugars and is a precursor to A- and B-type antigens: The A-type sugar chain has a fifth sugar, N-acetylgalactosamine, tacked onto it, and B-type chains have galactose. AB-type red blood cells sport both A and B antigens.
In 2007, scientists discovered bacterial enzymes that cut the terminal sugar off of most A and B antigens to convert them to O-type sugar chains (Nat. Biotechnol., DOI: 10.1038/nbt1298). Yet some recalcitrant types of A antigens, which have different linkages between sugars, resist pruning by any enzyme. Stephen G. Withers of the University of British Columbia decided to try his hand at directing the evolution of an enzyme to trim one of these stubborn antigens, which are found on less than 10% of type A red blood cells.
Withers’s group started with a family of enzymes that clips not just one sugar from A and B antigens (to make true type O blood), but three sugars, including an extra fucose and galactose. Withers’s group then mimicked natural evolution. They first mutated the enzyme’s genetic sequence and produced thousands of unique enzyme progeny. Then they picked the offspring that cut the linkages best and repeated the process.

They focused on genetic mutations at active sites in the enzyme that likely interact with the A antigen. After screening a total of about 10,000 enzymes, they obtained one with a 170-fold improved ability to cut antigens off recalcitrant-type red blood cells.
For this class of enzymes, says Henrik Clausen of the University of Copenhagen, “they have done a fantastic job of creating broader specificity and a more efficient enzyme.” But he cautions that a new problem arises with this particular strategy: By cutting off the three outermost sugars instead of just one, these enzymes expose an underlying carbohydrate marker that targets red blood cells for quick removal from the body. “This is not good for transfusion,” Clausen says. Enzymes that cut off just one sugar, adds Martin L. Olsson of Lund University, in Sweden, are more appropriate because they generate O-type red blood cells.
Withers acknowledges that red blood cells truncated by his enzyme could be cleared more rapidly from circulation. But if human testing shows that it is, he plans to use another enzyme to remove or cap the exposed chain. He also expects that his group can evolve the enzyme further to tackle other recalcitrant A subtypes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter