Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Porphyrins Run Rings Around Each Other
Supramolecular Chemistry: Concentric nanorings mimic photosynthetic complexes
by Mark Peplow
September 30, 2015

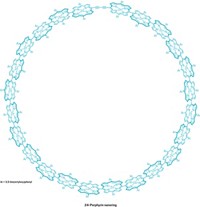
A pair of nanorings, one sitting inside the other, fit together like perfectly nested Russian dolls. The structure is not only strikingly beautiful but could offer a useful model for studying energy transfer within natural photosynthetic complexes. It also demonstrates how large nanorings can be built from components that self-assemble onto a smaller, template ring (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b07956).
“Energy migration between rings is quite a common theme in photosynthesis,” says Harry L. Anderson of the University of Oxford, whose team created the Russian doll complex. When purple bacteria harvest light, for example, energy cascades between rings of 9, 18, and 32 chlorophyll molecules that each bear a porphyrin group bound to a magnesium ion.
Anderson’s nanorings are a synthetic analog of these systems. The inner ring contains six aluminum-porphyrins, connected by dialkyne groups and supported by a scaffold of molecular spokes. The outer ring, made from 12 zinc-porphyrins, is tethered to the core by six Y-shaped ligands that bind to the zinc and aluminum centers.
The team initially created the concentric complex by building the rings separately and then combining them. But they found that the outer ring could be constructed by mixing chains of four zinc-porphyrins with the Y-shaped ligands and the inner ring. Once the zinc-porphyrin fragments had self-assembled around the inner ring, a palladium-catalyzed alkyne coupling reaction knitted them together into a single ring. Anderson says that bringing together 11 different components in this way represents a new way to create large molecular rings by templated self-assembly. “In principle, this could be a way of making bigger and bigger structures,” he says.
“Once you have seen the structures and the approach, then it looks obvious, but this work is a lot harder to analyze and execute than it looks,” says Jeremy K. Sanders of the University of Cambridge, who also works on supramolecular complexes that include metalloporphyrins.
Anderson’s team also probed the rings’ extended, delocalized pi-electron systems. When a photon hits the complex, it excites both rings’ pi-systems, but the inner ring reaches a higher energy state than the outer. That energy zooms from the inner to the outer ring in less than 40 ps, before being emitted as a lower-energy photon.
“When I saw the structure, I immediately thought of the light-harvesting system in nature, in which multiple porphyrin arrays harvest light energy,” says Makoto Fujita of the University of Tokyo, who has also developed concentric self-assembled structures. This similarity means that Russian doll complexes could be useful for testing theories about how energy migrates within photosynthetic complexes, or even lead to artificial light-harvesting systems.
Earlier this year, Anderson’s team unveiled small tubes that contained a pair of six-porphyrin rings stacked on top of each other. They now hope to use these as a template to create double-walled porphyrin tubes. These structures would have extended pi-electron systems running along and around each of the tubes and could exhibit unusual energy transfer processes or optical properties, Anderson says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter