The 2015 Nobel Prize in Chemistry has been awarded jointly to Tomas Lindahl of the Francis Crick Institute and Clare Hall Laboratory in England, Paul Modrich of Duke University School of Medicine, and Aziz Sancar of the University of North Carolina School of Medicine for their mechanistic studies of three different types of DNA repair.
Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nobel Prize
Tomas Lindahl, Paul Modrich, and Aziz Sancar Win 2015 Nobel Prize in Chemistry For DNA Repair
Researchers uncovered biochemical mechanisms that protect our genetic material
by Celia Arnaud
October 7, 2015
Our genetic material is constantly damaged. “As a rough estimate there are 10,000 DNA lesions per day per cell,” says Thomas Carell, who studies DNA repair at Ludwig Maximilian University in Munich, Germany. For example, every time DNA gets replicated, there’s a chance of the wrong base being inserted at a crucial spot. At the same time, chemicals or radiation can damage DNA bases.
“There’s no way to establish life based on such a fragile molecule without having sophisticated machinery to keep it in order,” Carell says. If these mistakes or damage don’t get fixed, they can lead to cancer or other diseases. “DNA repair is absolutely important to genome stability and of course to life.”
“I’m totally excited,” Carell adds. “The field is really deserving and all three winners have made extraordinary contributions.”
Lindahl focused on the repair system that kicks in when cytosine loses an amino group to form uracil. When this happens—which it does spontaneously on a routine basis—the uracil tends to pair with adenine instead of the guanine that cytosine is supposed to pair with. In 1974, Lindahl identified the first known repair protein, uracil-DNA glycosylase, which removes these erroneous uracil bases. Later, he discovered a second enzyme specific for damaged adenine. These turned out to be just two of a large family of proteins involved in so-called base excision repair. More than two decades later, Lindahl was able to recreate the human base excision repair process in vitro.



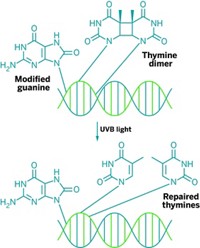
Sancar, a Turkish scientist working in the U.S., figured out the process by which cells repair UV damage by excising an entire nucleotide, rather than just the base. He found that an enzyme known as an exinuclease cuts out the damage, taking a piece about 12 nucleotides long in total. Then DNA polymerase fills in the gap, and DNA ligase stitches the pieces back together.
Modrich elucidated how cells repair mismatched bases in DNA. He showed that bacteria use an enzyme called dam methylase to mark damaged DNA with methyl groups that guide a restriction enzyme to cut in the right place. In 1989, he reported a system that could repair DNA mismatches in vitro. That system required DNA polymerase III, exonuclease I, and DNA ligase and was guided by DNA methylation. However, because methylation functions differently in bacterial and human systems, scientists still don’t know exactly how the system knows which strand needs to be repaired.
“The repair mechanisms that the Laureates have elucidated are all about the making and breaking of chemical bonds,” says Diane Grob Schmidt, president of the American Chemical Society, which publishes C&EN. “It’s chemistry in a biological context. It also illustrates the multidisciplinary aspects of this type of research and the grand challenges that we’re all trying to solve.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter