Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
EU’s REACH May Not Be Fostering Innovation In Industrial Chemicals
Law was intended to promote safer alternatives to toxic substances
by Alex Scott
January 4, 2016
| A version of this story appeared in
Volume 94, Issue 1

In a rousing speech during the late 1990s, Margot Wallström, who was then head of the European Commission’s environment division, chastised the chemical industry for failing to replace toxic substances. She declared that new legislation, the Registration, Evaluation, Authorisation & Restriction of Chemicals (REACH), would cut back the use of hazardous compounds and encourage the development of sustainable alternatives.
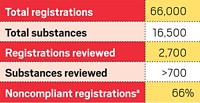
More than a decade on, many of REACH’s provisions are in effect. Industry has played its part by spending billions of dollars to characterize and register more than 6,000 substances with the European Union. As Wallström predicted, REACH in some cases has encouraged companies to substitute hazardous chemicals with safer alternatives. But there are other indications that REACH could be hampering innovation in the chemical sector.
Reach In A Nutshell
1. Companies must register with the European Chemical Agency all chemicals they make or import into the European Union. They must provide toxicity and other data to ECHA.
2. ECHA and EU member countries evaluate this information and determine whether a chemical’s health and environmental risks can be managed. If so, the substance generally is marketable in the EU, sometimes with restrictions.
3. Chemicals that are carcinogenic, mutagenic, toxic for reproduction, or exhibit a set of three characteristics—they are persistent, bioaccumulative, and toxic—get special treatment under REACH. They are classified as “substances of very high concern.” ECHA then places them on its “candidate list” for further review.
4. Regulators then determine whether these chemicals should get moved to ECHA’s Authorisation List. In general, substances on this list cannot be made, imported, or used in the EU after a specified date—unless the EU grants a company special permission to do so. —Cheryl Hogue
The European Chemical Agency (ECHA), though, has no doubt that greener alternatives are increasingly trumping hazardous chemicals. Its assertion is based on a growing body of anecdotal evidence to show that REACH is driving innovation. “Examples of substitutes are easily found,” says Jack de Bruijn, director of risk management for ECHA.
One instance is the trend among chemical manufacturers to develop a greater number of alternatives for certain hormone-disrupting phthalate plasticizers, says Washington, D.C.-based advocacy group the Center for International Environmental Law. According to the group, the number of patents filed annually for phthalate substitutes increased from one to around five when temporary EU phthalate controls were introduced in 1999. They increased from seven to about 15 in 2006 when the EU adopted REACH. And they rose to 20 soon after 2008 when four phthalates were classified under REACH as substances of very high concern (SVHCs).
“Spikes in the patenting of phthalate alternatives clearly correlate with the timing of new laws to protect people,” Baskut Tuncak, an attorney for the center, notes in a 2013 study on REACH and innovation.
ECHA, however, has no clear view about REACH’s impact on hazardous industrial chemicals overall. The picture is a complex one, with an array of factors affecting innovation, the agency’s de Bruijn says. It is difficult to identify whether a novel alternative chemical was developed specifically as a substitute for a hazardous chemical, he says.
In the meantime, ECHA is encouraging the development of greener substitutes by dissuading chemical companies from keeping their most hazardous chemicals on the market. ECHA is doing this primarily by placing SVHCs on its so-called candidate list. This action puts a chemical in a queue for highly detailed scrutiny by the European Commission, the EU’s executive branch.
ECHA started putting SVHCs on the candidate list in February 2011. Now, there are 163 substances listed. More are being added regularly.
Because it is public, the candidate list is influencing entire supply chains. Increasingly, large multinational consumer products companies have stopped using chemicals on the list. Companies that are phasing out—or have already eliminated—SVHCs from their products include Swedish household products firm Ikea, Sony Mobile, Danish food retailer Coop, and members of the industry program Zero Discharge of Hazardous Chemicals, whose members include Adidas, Gap, and Nike.
As a result, REACH’s impact extends to countries outside the EU, says Matti Vainio, head of ECHA’s Risk Management Implementation Unit. That includes the U.S., where legislation has passed both houses of Congress to reform the federal chemical control law, the Toxic Substances Control Act, to require chemical reviews that would bear similarities to REACH evaluations (see page 6).
Substances on the candidate list can ultimately wind up on the EU’s Authorisation List. Chemicals on this latter list are banned. But companies can request that the government grant them permission to continue production, import, and use of a substance in certain applications.
Companies have applied for permission to use some of the substances phased out or set to be phased out under REACH
Sixty-eight applications received for
1,2-Dichloroethane (ethylene dichloride)
Bis(2-ethylhexyl) phthalatea
Chromium trioxide
Diarsenic trioxidea
Dibutyl phthalatea
Hexabromocyclododecane
Lead chromates (3 substances)
Sodium chromate
Sodium dichromate
Trichloroethylenea
No applications received for
2,4-Dinitrotoluene
4,4’-Diaminodiphenylmethane (MDA)
5-tert-butyl-2,4,6-trinitro-m-xylene (musk xylene)
Benzyl butyl phthalate
Diarsenic pentaoxide
Diisobutyl phthalate
Tris(2-chloroethyl)phosphate
Applications are expected to be received for
2,2’-dichloro-4,4’-methylenedianiline
Acids generated from chromium trioxide and their oligomers
Ammonium dichromate
Arsenic acid
Bis(2-methoxyethyl) ether (diglyme)
Dichromium tris(chromate)
Formaldehyde, oligomeric reaction products with aniline (technical MDA)
Pentazinc chromate octahydroxide
Potassium chromate
Potassium dichromate
Potassium zinc chromate hydroxide
Strontium chromate
a European Commission has issued a decision for some applications.
SOURCE: ECHA
Only 31 substances are currently on the Authorisation List. So far, ECHA has received applications for continued use of only 12 of them. “It is evident from the applications that companies have plans to substitute but that this takes some time,” adds Vainio. Continued-use extensions give companies the time to find a suitable substitute, Vainio says.
Erwin Annys, REACH and chemicals policy director for Cefic, Europe’s leading chemical industry association, rejects the notion that there have been no substitutions as a result of REACH. For 50% of the substances on the Authorisation List, ECHA has received no application for continued use, he says. This means that chemical firms have no intention of continuing to produce such substances. “Meaning they have been substituted already,” Annys says. ECHA’s de Bruijn agrees.
Additionally, in a significant number of REACH registration packages, called dossiers, industry has proposed novel greener chemicals to substitute hazardous ones. “Hence, innovation is taking place in Europe,” Annys says.
As an example, Switzerland-based industrial products firm Oerlikon was inspired by REACH to develop a replacement for an electroplating process that uses carcinogenic hexavalent chromium. Oerliken’s technology combines physical vapor deposition with UV-lacquering, thereby avoiding the need to use any toxic agents such as Cr(VI).
“We see that REACH is having a big effect on driving alternatives,” says Rüdiger Schäfer, head of sales and technology for Oerliken. He suggests that the shorter the time regulators authorize the continued use of a blacklisted chemical, the faster companies will make the switch, he says.
The Comission has the final word on which substances are added to the Authorisation List. The process for placing chemicals on this list is still new and involves the compilation of a lot of data and other documentation.
Adding substances to the Authorisation List has taken longer than the Comission had expected, de Bruijn says. “Once we are more experienced, we will see a quicker flow,” he adds, noting that the Comission expects to further streamline the process in 2016.
That will please environmental groups such as Sweden-based ChemSec, which sees the threat of banning substances as vital for encouraging chemical firms to develop greener alternatives.
In some cases, though, REACH could be too lenient on the chemical industry, ChemSec argues. As an example, Canada-based Dominion Color Corp., the world’s largest producer of lead chromate pigments, is seeking permission to continue selling the SVHC lead chromate, a bright yellow pigment, for use in road marking paints. The original phaseout date for lead chromate was May 21, 2015.
ECHA has recommended that lead chromate be authorized for another 12 years. The Commission is now due to make a final judgement.
But the substance, which contains both lead and Cr(VI), is highly toxic and has been voluntarily phased out in Sweden for about 20 years, says ChemSec chemicals and policy specialist Frida Hök. It should now be banned across the EU, she argues.
Overall, though, ChemSec is “absolutely convinced” that the SVHC list and the authorization process are encouraging companies to develop greener chemicals, Hök says.
The Comission shares this conclusion. At the end of a two-day conference on lessons learned from REACH authorization held toward the end of 2015, Bjorn Hansen, head of the chemicals unit for the Comission, said that the process “provides pressure on industry to substitute toward safer substances.” Nevertheless, during the conference the issue of whether SVHCs are being progressively replaced with safer alternatives came up repeatedly. No clear answer emerged.
In an attempt to further encourage the substitution of hazardous substances, ChemSec for several years has published its SIN (Substitute It Now) list of chemicals that the organization says fulfil the criteria for SVHC classification. Containing 844 chemicals, the SIN list includes many chemicals not on ECHA’s candidate list. A number of companies have a policy of not using chemicals on the SIN list, Hök says.
In recent months ChemSec also has rolled out SINimilarity, a free online tool designed for nonchemists working in companies that use chemicals. The tool helps them identify whether the chemicals their companies use have a structural similarity to a known hazardous substance.
However, many still maintain that REACH is suppressing innovation. This position is backed up by a survey undertaken by the Comission in early 2015 of 1,500 small and medium-sized companies. It found 35% of firms stated that REACH is negatively affecting their capacity to innovate. Just 10% of companies surveyed indicated that REACH is having a positive impact on innovation.
Commenting a few months ago on the findings of the survey, Antti Peltomäki, the deputy director general of the Comission’s industry directorate, said, “Experience so far suggests that the outcomes are not as positive as we would like to have seen.” Substituting chemicals on the candidate list does not equate to innovation, he adds.
Peltomäki suggests the reduced innovation among smaller chemical firms could stem from the relatively high burden that small companies bear for complying with REACH. He calls for a balance among health, innovation, and competitiveness.
Peltomäki’s comments were leapt upon by the European Environment Bureau, a federation of more than 140 European environmental organizations. His remarks “lack any factual support,” bureau Secretary General Jeremy Wates wrote in an open letter to Industry Commissioner Elzbieta Bienkowska. “Most studies performed to date show the great benefits of regulating hazardous chemicals for innovation as well as for health and the environment,” Wates added.
In another indication that REACH may not be effective at driving innovation, users of certain chemicals that are on the Authorisation List and set to be phased out say that they will have to move production out of the EU because no substitutes are available. As an example, Sweden-based recreational vehicle producer Dometic says there is not yet a replacement for sodium chromate, a carcinogen the firm uses as an anticorrosion agent for its refrigerators. This substance is due to be phased out in September 2017.
Dometic claims a substitute chemical won’t be available until 2018 and that the company won’t be able to fully phase out sodium chromate until 2029. Dometic plans to build a $38 million plant in Zhuhai, China, and relocate production from Europe. This puts at risk the jobs of 55 of its employees in the EU if the use of sodium chromate is not extended there.
ChemSec’s Hök says this case is just industry posturing. “It is more of a scare tactic. Of course, some will move out—but most will not,” she says.
In some cases where substitutes for chemicals on the Authorisation List are not readily available, the Comission is funding the development of suitable alternatives. As an example, NANOFABS, a consortium of European research institutes and companies funded by the Comission in 2014, completed the development of a flame retardant for acrylonitrile-butadiene-styrene compounds used in electronics. It is designed to replace halogenated flame retardants on the Authorisation List. The substitute consists of a phosphorous-based material, which has better environmental performance than halogenated flame retardants and meets requirements for fire prevention. NANOFABS partners include Italy’s Politecnico Di Torino University and German plastics compounder Sitraplas.
Larger European chemical companies, such as BASF, Evonik Industries, and Lanxess, though, say that they have felt little adverse impact from the introduction of the REACH candidate list. “Very little has been phased out” at Lanxess, says Anno Borkowsky, CEO of the company’s Rhein Chemie Additives business.
As a result of REACH, no chemicals that BASF makes have been phased out either. “REACH is an important step to harmonize chemical legislation in the EU, but it has no driving effect with regard to the development of new chemicals,” BASF tells C&EN.
The impetus for more sustainable products is not from REACH but from customers, BASF and Evonik Industries say.
Wallström, along with today’s EU regulators, would be far from happy to see a REACH that hampers innovation in small chemical companies and has little tangible effect on innovation among larger chemical firms. But despite the Comission’s best efforts, this might be the case. ◾



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter