Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Movers And Shakers
A conversation with Christina Smolke
The synthetic biology pioneer discusses how she reprogrammed yeast to produce opioids.
by Mark Peplow, special to C&EN
March 21, 2016
| A version of this story appeared in
Volume 94, Issue 12
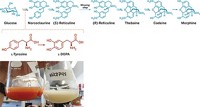
The world’s entire supply of opioid painkillers begins with the opium poppy (Papaver somniferum). Every year, about 250,000 acres of the crop is legally grown for its opiate compounds, which are extracted and converted into analgesics such as codeine and hydrocodone. Bioengineer Christina Smolke of Stanford University thinks it’s time for a new approach. Last year, she developed yeast that turns glucose into the opioids thebaine and hydrocodone (Science 2015, DOI: 10.1126/science.aac9373). The work could offer a more efficient route to the vital drugs through industrial fermentation. Mark Peplow talked with Smolke about the promise—and the challenges—of her synthetic biology project.
Vitals
Hometown: Torrance, Calif.
Studies:
B.S., University of Southern California, 1997
Ph.D., University of California, Berkeley, 2001
Professional highlights:
California Institute of Technology, 2003–10
Stanford University, 2009–present
2004 MIT Technology Review TR100 innovator under 35
Favorite molecule: Theobromine
Favorite hobbies: Hiking, yoga
What’s wrong with farming opioids?
There are a lot of disadvantages in using agriculture to make medicines. Plants accumulate limited quantities of the compounds you want and make a limited range of end products, which might not be the best compounds for a particular therapy. So let’s take the synthetic pathways and extend and modify them to produce novel compounds. Opium poppies make mostly morphine and thebaine. There’s a real potential here to go beyond what nature gives us and reduce some of the negative side effects associated with these compounds.
How did you develop a yeast strain to make thebaine and hydrocodone?
First we identified enzymes that help convert glucose into a morphinan scaffold. We were not limited to enzymes from opium poppies. We took enzymes from other poppies, from bacteria, and even from rats, which provided an enzyme to make the intermediate
Then we made the DNA that gives the yeast the instructions to produce those enzymes, more than 20 of them. That’s a lot of enzymes that the yeast doesn’t ordinarily make that you have to coordinate.
Several groups, including your own, had re-created various segments of this pathway in yeast during the past couple of years. Was it just a case of stitching them all together?
In addition to integrating everything, we had to optimize how much sugar the process required, and we filled in a step that was missing in the middle of the synthesis—transforming (S)-reticuline to (R)-reticuline. We looked at the genome of the poppy and other plant species to identify variants of the enzyme responsible for that transformation and ended up using one from the Iranian poppy.
The enzyme immediately before that step was also inefficient. We had to do some protein engineering on the enzyme to improve it so that we could accumulate more downstream product. When you have that long a pathway, each step has to be efficient enough to accumulate enough product at the end.
Is this a pathway that you could actually scale up for manufacturing drugs?
You’d be foolish to try to scale up this yeast strain for production. It produces so little of the product that the economics wouldn’t make sense. The yield was micrograms per liter of fermentation broth, which is far from where you want to be for commercial production—low grams per liter. The point of the work was to show proof of principle.
The next stage is optimizing how the yeast grows, opening up bottlenecks in the pathway, and improving the fermentation in general. If you look at what has happened in biotechnology in the past five years, it’s feasible to achieve that level of optimization. We have started a company, Antheia, which is now increasing the yields.
Could these strains be used to “home-brew” opioids for illicit use?
How you optimize the strain plays a big role in which compounds you end up making and how much of them. Even if you had the optimized strain, it wouldn’t produce much of the compound—if at all—unless the conditions were right. The strains would also be tightly regulated, both for intellectual property reasons and because of drug enforcement.
So my own take is that home-brew is less of a concern. We have a problem with abuse of opioids, but it’s not clear that the way we make the drugs determines how much gets into the black market. Other things also impact this—policies toward prescription drugs and how we help addicts, for example—so the conversation has to go a lot further than the technology.
Will a synthetic biology approach such as your yeast ever compete with conventional organic synthesis?
You need a combination. You want to use biology to get to the complicated molecular scaffolds, for example. There are things that biology is going to do really well and things that chemistry does really well. You want to leverage both.
Mark Peplow is a freelance writer. This interview first appeared in ACS Central Science:http://cenm.ag/smolke.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter