Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Ceramic-graphene composite shows promise as battery electrode
Material combines silicon oxycarbide’s large capacity for storing lithium and graphene’s high electrical conductivity
by Mitch Jacoby
April 11, 2016
| A version of this story appeared in
Volume 94, Issue 15
Replacing the graphite anode commonly used in lithium-ion batteries with ones made of silicon or graphene, which have high lithium uptake capacities, could substantially boost the batteries’ charge capacity, in principle. In practice, however, silicon electrodes swell and shrink with each charging cycle, causing them to crack and fail quickly. Graphene electrodes also quickly lose their capacity to store and release lithium ions.
By combining the two candidate electrode materials, Gurpreet Singh and coworkers at Kansas State University may have hit upon a solution (Nat. Commun. 2016, DOI: 10.1038/ncomms10998).
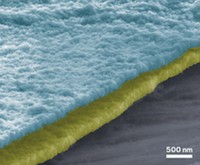
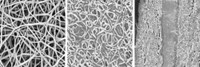
Starting with a siloxane precursor, the team used a simple heat treatment to form micrometer-sized particles of silicon oxycarbide (SiOC), a glass-ceramic with an open polymer-network-like structure. They then used a standard method to prepare a form of graphene known as reduced graphene oxide (rGO) and finally formed free-standing sheets of an SiOC-rGO composite “paper” via vacuum filtration.
Battery tests show that the low-cost, lightweight paper electrode exhibits a high charge capacity and stands up to repeated cycling. The electrode showed nearly no loss of charge capacity and no sign of mechanical failure even after 1,020 charging cycles, the team reports.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter