Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Safety
How educators are teaching students to assess risk in the lab
Safety experts and professors share their approaches for moving beyond simple lab safety rules to teach students new skills
by Jyllian Kemsley
April 13, 2016
| A version of this story appeared in
Volume 94, Issue 16
“A rules-based approach to chemical safety education and information is inadequate,” said Ralph Stuart during a symposium at last month’s American Chemical Society national meeting in San Diego.
Stuart, the environmental safety manager at Keene State College, recommends something better. Educators should focus on teaching their students hazard and risk assessment skills, Stuart and others believe. This view is bolstered by guidelines issued last year by ACS for two-year college and bachelor’s degree chemistry programs: “Classroom and laboratory discussions need to stress safe practices and should actively engage students in the evaluation and assessment of safety risks associated with laboratory experiences,” say the bachelor’s degree guidelines.
Educators and safety experts shared their approaches and tools for incorporating safety assessments into educational lab curricula at the meeting in San Diego during a Division of Chemical Health & Safety symposium organized by Stuart and Samuella B. Sigmann, a senior lecturer at Appalachian State University.
“We’ve always been concerned about safety in the laboratory for our academic courses, but that’s translated into a few minutes of cautionary comment when giving a prelab lecture. We need to do a better job,” said Lawrence Tirri, a professor of chemistry and biochemistry at the University of Nevada, Las Vegas, when introducing his department’s plans.
First, UNLV chemistry and biochemistry first-year graduate teaching assistants, who teach all of the school’s general and organic chemistry labs and assist with upper-level labs, will get more intensive training before classes start in the fall. Because the incoming graduate students have various backgrounds, Tirri wants to use the training to ensure they all meet a basic level of safety awareness and practice. “We’ll talk about chemical hygiene and safety culture concepts and go through the tools available for hazard identification and assessing risks,” Tirri said. “We want to make sure they understand what our department expects of them when they go into the labs and what they should convey to their students.”
Then, for the undergraduates taking lab courses, Tirri aims to start including the concepts of hazard identification and risk assessment in the curriculum. Topics will include consideration of the hazards of different quantities or concentrations of various materials and safe handling practices. Students will learn basic concepts in general chemistry and engage more deeply as they move to upper-level courses. Lab quizzes and exams will include safety-related questions. In their senior year, students will have to write a full hazard analysis report.
“We’re just starting this journey,” Tirri said. “We’re hoping that it’s going to help students develop their thinking and be more aware of what’s going on around them, not just in the laboratory but in their everyday lives.”
At Appalachian State, deeper engagement with safety occurs at the junior and senior undergraduate level, Sigmann told C&EN. At the junior level, students must take an introductory course on research that covers information literacy, ethics, grant writing, and safety. Sigmann teaches the safety component, during which she covers types of hazards, components of risk, and where to find safety information. She also goes over how to do a hazard analysis, using changing a lightbulb as an example: As a class exercise, she breaks down the task into steps and goes through the hazards, risk level, and safety controls for each step.
“The purpose is not to make students responsible for everyone else’s safety behavior but to give the class a sense that we’re all accountable for safety.”
-P. J. Alaimo, professor of chemistry, Seattle University
Then the students complete their own hazard analysis for a laboratory procedure. “The hardest thing for them to wrap their heads around is dividing a process into steps and determining the risk for each step,” she said. “They tend to want to combine or skip steps, such as pouring 30 mL of nitric acid into a beaker, skipping taking that 2.5-L bottle out of the cabinet and transporting it. But transporting the bottle is one of the highest hazard steps—if you drop it and it breaks, it’s likely a hazmat situation.”
Students doing research projects in their senior year must also do a hazard analysis as part of their research proposal. “Students are very receptive,” Sigmann said about the hazard assessment assignments. “They stop in for help and say, ‘I’ve never thought about this before.’ ” The skills her students acquire by doing these assessments stay with them as they transition into internships at government labs or jobs elsewhere, Sigmann said.
Instructors at some schools are incorporating more intensive hazard assessment into their labs at the general and organic chemistry levels. Last fall, Melissa Anderson of Pasadena City College taught an honors general chemistry lab in which students did independent projects. On the first day of a multiweek titration unit, she introduced students to the various kinds of chemical and physical hazards, had them do an activity involving safety data sheets, and then had them do a titration with caustic sodium hydroxide.
Subsequently, as part of planning their independent projects, Anderson’s students completed a full hazard analysis using a worksheet Anderson developed from the ACS publication “Identifying and Evaluating Hazards in Research Laboratories.”
The worksheet guided students through analyzing their reagents, equipment, and processes. It also asked students to consider whether substituting a different chemical or process could achieve their goals with fewer hazards. “Those questions were hard for them,” Anderson told C&EN. But she sees that challenge as an opportunity to get the students thinking more deeply about the fundamental purpose of their projects, what variables they can change for safety reasons, and which ones they can’t.
This semester, Anderson is teaching chemistry to students in allied health programs such as nursing and dental hygiene, and she is thinking about how to build safety into that program. She wants to focus on the Globally Harmonized System of Classification & Labelling of Chemicals, which classifies chemicals by their hazard and hazard severity. “I think it provides a really useful framework for helping students think about hazards,” Anderson said. “You want to give them tools to reach an informed perspective instead of thinking that everything is always safe or always dangerous.”
Seattle University has also been working to increase its safety education starting from general chemistry, through what the chemistry department calls a “safety teams” approach that started several years ago (J. Chem. Educ. 2010, DOI: 10.1021/ed100207d). Beginning in their second quarter of general chemistry, students are divided into teams of three or four students, separate from their lab experiment partners. For one lab each quarter, each team is assigned to be an extra set of eyes in the lab: The teams do a prelab inspection to make sure all students have appropriate clothing and personal protective equipment (PPE), backpacks are out of the way, and safety equipment is available. During the lab, each safety team member periodically walks around to ensure that PPE is still in place, hood sashes are at the right level, reagents are capped, and waste bottles are not overfilled. At the end of the lab, safety team members ensure that everything is put away, benches and balances are clean, and equipment is in good condition.
At the start, “some students are hesitant to call out their peers,” Andrea Verdan told C&EN. “But once the first group gets through, it goes smoothly.”
“The purpose is not to make students responsible for everyone else’s safety behavior but to give the class a sense that we’re all accountable for safety,” said chemistry professor P. J. Alaimo.
In Seattle’s organic chemistry labs, instructors keep up the safety teams approach, but they add a responsibility: Teams must also do a hazard assessment of the lab for which they’re responsible. The assessment tasks include creating a safety handout, submitting it to the instructor to review in advance, and presenting a safety briefing to classmates at the start of the lab. “Students really feel like they’re part of something important,” Alaimo said. Also, “we never ever saw students having safety conversations with each other prior to implementation of this program. Now it’s almost as common as ‘What do we do next?’ ”
The students’ training in general and organic labs then carries over to upper-level labs and research projects. Although Seattle professor Ian Suydam doesn’t have students do a safety briefing for his physical chemistry lab—the concerns are more instrumental rather than chemical and harder for the students to research in advance—he believes that the earlier experiences prime students to think and ask about safety concerns.
And when students start research projects, “I know exactly what they’ve seen before and that makes it easier to train them in my lab,” Suydam said. “I think it would be so much more work and I’d be so much more worried if they hadn’t had that introduction.”
“Our students know they can’t run a reaction without knowing the reagent’s properties, or they can’t decide how to measure something out unless they know its phase and toxicological concerns,” Alaimo said. “We want them to be able to walk into grad school or a job ready to go.”
A way to assess risk
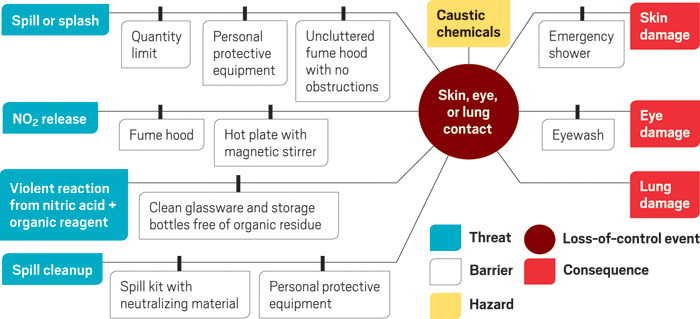
One risk assessment tool that could help students and others think through their experiments is the “bowtie” method. This method was presented at the ACS meeting in San Diego by Mary Beth Mulcahy, an investigator at the Chemical Safety Board, and Chris Boylan, head of the safety systems risk section at risk management company DNV GL. The example shown here is for preparing a stock solution of copper(II) nitrate by using concentrated nitric acid to digest copper wire.
The general concept of the bowtie is to identify a particular hazard of an operation, such as using a corrosive reagent. Then you predict a critical event in which you’ve lost control of that hazard, such as spilling the reagent. The hazard and loss-of-control event go in the center of the diagram.
You work right from the center to identify and list potential consequences of the loss-of-control event, such as chemical burns and lab damage, as well as the barriers that would help prevent or mitigate the consequences. You work left from the center to identify and list the threats that could lead to the event, such as a spill or organic residue left in a beaker, as well as the barriers that would help keep the threat from turning into the full-blown event.
A bowtie diagram by itself is “a tool for communication, not hazard identification,” Boylan emphasized, although a bowtie exercise could include checking safety data sheets or other resources to identify hazards.
\n





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter