Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
For Lewis acids and bases, a little frustration goes a long way
Chemists’ understanding of unquenched acid-base pair reactivity expands to include metal oxide surfaces
by Stephen K. Ritter
April 25, 2016
| A version of this story appeared in
Volume 94, Issue 17

Chemists have long known that catalytic Lewis acidic and Lewis basic sites on metal and metal oxide surfaces help spark a range of reactions that are important in industrial chemical and fuel production. Yet researchers still have no general theory available to predict all the mechanistic nuances of how these electron-deficient and electron-rich sites lead to that reactivity.
As a case in point, a research team led by nanochemistry specialist Geoffrey A. Ozin and computational materials engineer Chandra Veer Singh of the University of Toronto has recently uncovered a previously overlooked phenomenon involving cooperative acid-base reactivity on the surface of the solid photocatalyst indium oxide. In a set of structural and kinetic studies, the researchers show that closely situated Lewis acidic and basic sites on the solid catalyst act like solution-based molecular systems known as frustrated Lewis pairs (FLPs).
Ozin, Singh, and their colleagues say this heterogeneous surface analog of the homogeneous FLP effect could lead to better design of catalysts for refinery processes involving small gaseous molecules such as H2, CO, and CO2. In particular, they see a new way forward for the future large-scale use of sunlight and CO2 for making fuels and chemicals.
The Toronto team’s observations and analysis should inspire both academic and industrial chemists, says Zaiku Xie, general director of research at Chinese petrochemical firm Sinopec. “Surface FLPs not only provide an alternative mechanism to transform CO2 into chemicals in an effective way, but they also enrich and deepen our understanding of heterogeneous catalysis,” he says. Xie thinks this concept will help guide the discovery of more effective and selective heterogeneous catalysts to activate C–O and C–H bonds.
That Lewis acidic and basic sites in metal oxides might be working cooperatively reminded Ozin and Singh of the work of their University of Toronto chemistry colleague Douglas W. Stephan. In 2006, while at the University of Windsor, Stephan’s group built on earlier observations in Lewis acid-base chemistry to design new metal-free molecular systems with enhanced catalytic activity.
Lewis acids and bases are one of chemistry’s most fundamental concepts. An electron-deficient Lewis acid readily accepts a spare pair of electrons from an electron-rich Lewis base, with the acid-base pair typically forming a neutral adduct. The new twist recognized by the Stephan group is that when the Lewis acid and base are each dressed with bulky substituents that prevent them from forming a close relationship—denying their ability to form a neutral adduct—the sterically encumbered pair becomes “frustrated.” The unquenched FLP garners penned-up reactivity, comparable to that of an organometallic catalyst, which can be used to a synthetic advantage.
Stephan’s original FLP, which combined an electron-deficient bulky borane with an electron-rich bulky phosphine, was the first non-transition-metal system known to reversibly bind and cleave H2. Since that time, Stephan’s group and others have created a variety of different types of FLPs, including molecules in which acidic and basic moieties are separated by a spacer group within the same molecule or in which one of the partners is immobilized and the other is not. In addition, researchers have used metal-oxo complexes as a base in an FLP, showing that incorporating a transition metal can have a further synergistic effect on catalyst activity.
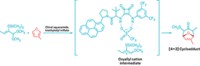
The new study by Ozin, Singh, and coworkers provides the first example of extending the FLP concept to surface chemistry. The researchers literally and figuratively shined light on the subject by carrying out experimental and computational studies on the photocatalytic behavior of pristine and intentionally defected indium oxide, In2O3. Indium oxide is used as a light-driven catalyst and is a popular photoactive material used in flat-panel displays, solar cells, and light-emitting diodes.
They observed that defect-ridden material containing oxygen vacancies and hydroxyl groups, denoted as In2O3–x(OH)y, provides enhanced activity for the reverse water-gas shift reaction, CO2 + H2 → CO + H2O. This reaction and related ones they have studied, such as the Sabatier reaction, CO2 + 4H2 → CH4 + 2H2O, are part of an array of industrial reactions involving simple gaseous molecules that are critical in producing ammonia, methanol, formic acid, and methane to make chemicals and fuels (see page 30).
The defects involve Lewis acidic unsaturated indium atom surface sites created by oxygen vacancies in the In2O3 lattice. The vacancies are situated immediately adjacent to Lewis basic In–OH groups that form when the precursor In(OH)3 is dehydroxylated under controlled temperature conditions. The number of hydroxyl groups is key to optimizing the catalytic activity.

The Ozin-Singh team found that the enhanced activity only takes place at the adjacent defective sites when irradiated by light. The defects become more acidic and more basic when excited, Ozin explains, which lowers the activation energy of the rate-limiting CO2 reduction step. The effect isn’t observed for defect surfaces containing only oxygen vacancies or only hydroxide groups or when the defects are not close together, and it barely occurs in the dark (J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.5b10179).
Chemists know that surface defects, such as oxygen vacancies and hydroxyl groups, and the coupling of different catalytic sites via diffusion of reaction intermediates can be important in catalytic activity, Ozin notes. This phenomenon is also at work at acidic and basic sites confined inside the pores of zeolites and on the surfaces of small clusters of metal atoms or metal oxides in nanoparticle catalysts.
“What we have here is not simply a case of rediscovery of surface acidity and basicity in catalysis,” Ozin suggests. “Instead it represents a new way of thinking about old ideas in heterogeneous catalysis. The recognized symbiosis between molecular FLPs and surface FLPs should now make it possible to rationally design and chemically tailor the surface structure and composition of nanoscale metal oxides to optimize catalytic activity.”
“It’s certainly gratifying to see the frustrated Lewis pair concept we articulated based on main-group reactivity finding broader applications in transition-metal chemistry,” Stephan says.
The most remarkable consequence of FLPs so far, Stephan notes, has been their use as catalysts for hydrogenation of a range of organic substrates. For example, chemists have harnessed FLPs to hydrogenate imines, carbonyls, and silyl ethers as well as to hydrogenate polyaromatic hydrocarbons, olefins, and alkynes.
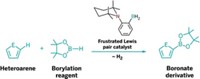
Researchers have also used FLPs for metal-free hydrogenation of aromatic compounds such as anilines, which is hard to do even with the best transition-metal catalysts. They’ve also used them to activate C–H bonds of furans and other heterocycles to form boronates for cross-coupling reactions. Going beyond H2, Stephan’s group and others have used FLPs to cooperatively bind NO, SO2, NO, and CO2 in metal-free reactions to functionalize organic molecules.
Ozin and Singh hope their revelation about surface FLPs will add to that growing list of FLP accomplishments. In particular, they think the concept will help lead to a more sustainable future by allowing scientists and engineers to develop practical methods for using solar energy to convert CO2 greenhouse gas emissions into value-added chemicals and fuels.
“Arguably the most important challenge facing those who wish to harness solar energy and at the same time diminish the amount of anthropogenically produced CO2 is to achieve a photocatalytic Sabatier reaction,” comments John Meurig Thomas, a heterogeneous catalysis expert at the University of Cambridge. “The next most important challenge is to discover efficient ways of effecting a photocatalytic reverse water-gas shift reaction.”
Thomas adds that the discovery of surface FLPs constitutes a significant advance for both challenges. “This work not only achieves commendable conversions with various defective forms of indium oxide photocatalysts, but it also introduces a promising theoretical framework that can be built upon to yield yet more powerful photocatalysts.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter