Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Coating plus doping improves performance of promising material for lithium-ion batteries
Treating cathode compound with iron via atomic layer deposition boosts capacity and extends lifetime
by Mitch Jacoby
May 16, 2016
| A version of this story appeared in
Volume 94, Issue 20

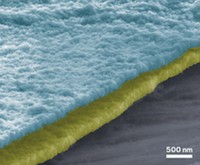
Atomic layer deposition (ALD), a well-studied layer-by-layer method for depositing thin films on solids, is prized for its ability to form ultrathin films that conform perfectly to oddly shaped and tough-to-coat solid surfaces. A study finds that not only does ALD coat materials with a film-forming compound, but also it can penetrate the solid, doping the material. This combination of coating and doping can improve lithium-ion battery materials (Sci. Rep. 2016, DOI: 10.1038/srep25293).
LiMn1.5Ni0.5O4 (LMNO) has drawn significant attention as a candidate Li-ion battery cathode material because of its low cost, high theoretical charge capacity, and high operating voltage relative to other commonly studied cathode materials, including LiMn2O4, which has been used in plug-in hybrid electric vehicle batteries. In practice, however, unwanted electrochemical reactions cause LMNO’s charge capacity to diminish quickly.
A team led by Xinhua Liang of Missouri University of Science & Technology reasoned that treating LMNO with iron oxide, an electrochemically active compound, via high-temperature ALD would coat and dope LMNO and improve its properties. It worked.
Compared with naked LMNO, the ALD-treated samples exhibited 25% higher charge capacity and retained roughly 93% of their capacity even after 1,000 charging cycles.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter