Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Start your engines
As the Volkswagen scandal showed, building fuel-efficient, low-emitting vehicles is no easy task
by Melissae Fellet, special to C&EN
June 6, 2016
| A version of this story appeared in
Volume 94, Issue 23

Volkswagen is still reeling from the U.S. Environmental Protection Agency’s revelation that the carmaker had altered diesel engines to beat emissions tests, which caused immediate consumer outrage. The agency reported that some diesel-powered vehicles made by Volkswagen and its subsidiaries Audi and Porsche contained software that lowered emissions during tests but not during normal driving. On the road, some vehicles emitted up to 40 times as much NOx—a combination of nitric oxide and nitrogen dioxide—as allowed.
Short-term exposure to nitrogen dioxide is linked to airway inflammation and increased asthma symptoms. In the atmosphere, nitrogen dioxide contributes to the formation of ground-level ozone and particulate matter, which create further negative health effects. A recent study estimated that excess NOx released by the altered Volkswagen cars in the U.S. could cause 46 additional expected deaths and additional damages totaling $430 million, compared with if emissions regulations were followed (2015, DOI: 10.1021/acs.est.5b05190). In Europe, however, excess NOx from the altered cars may have little impact: High NOx emissions already plague the region because it has weaker emissions regulations and more diesel passenger cars than the U.S.
Balancing NOx emissions control with fuel efficiency is a tough nut to crack. Often the most efficient operating conditions for a diesel engine make it difficult to remove NOx in the exhaust. “I’m certainly not justifying what Volkswagen did, but if the solution was easy, hopefully they wouldn’t have done it,” says Michael P. Harold, a chemical engineer at the University of Houston. Especially as fuel efficiency standards and NOx emissions limits tighten, experts say NOx control systems need big improvements to keep pace. Central to this are improvements to the NOx-clearing catalysts and systems for diesel vehicles. The race is on for a vehicle that saves fuel and releases clean exhaust, too.
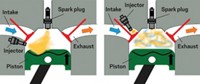
Efficiency versus emissions
When engines burn fuel, they create a hot mixture of gases that expand and push a piston in a cylinder, converting the fuel’s chemical energy to mechanical energy. Diesel engines are 15 to 20% more efficient than conventional gasoline engines in part because they contain excess air during combustion. The oxygen-rich mixture of combustion products in a diesel engine expands more and transfers more energy into pushing the piston than the products of a gasoline engine, generating more mechanical energy for a given amount of fuel. After combustion, the temperature inside both diesel and gasoline engines is high enough to drive the reaction of nitrogen in the air to NOx.
That’s when emissions control systems need to kick in. If the systems work well, then the engine can run efficiently while the majority of NOx generated will be removed from the exhaust and converted back into nitrogen. But the excess oxygen present in a diesel engine creates an oxidizing environment that makes it difficult to chemically reduce NOx back to nitrogen. Even the best-performing NOx emissions systems sap fuel efficiency and add to the cost of a vehicle.
The most common NOx after-treatment system used in diesel engines, called selective catalytic reduction (SCR), contains a catalyst that selectively combines NOx and ammonia to produce nitrogen. Urea, supplied by an onboard tank, decomposes into ammonia, which is injected into the exhaust system. But the biggest problem is that the zeolite catalysts in these systems do not work at temperatures below about 200 °C. Such temperatures occur during cold starts or low-load conditions, or in the cooler exhaust of more fuel-efficient engines. Over the lifetime of a vehicle, the majority of its NOx emissions come during cold-start temperatures.
“The challenge to the community is to develop a catalyst that gives 90% NOx conversion at 150 °C,” says Rajamani Gounder, a chemical engineer at Purdue University, referring to a target set by U.S. DRIVE (Driving Research & Innovation for Vehicle Efficiency & Energy Sustainability), a partnership between U.S. automakers, energy companies, and the Department of Energy. He and a team of researchers at Purdue, the University of Notre Dame, and Argonne National Laboratory are studying exactly how one zeolite catalyst at the heart of the emissions control works. The catalyst is based on the aluminosilicate form of chabazite, a naturally occurring, porous mineral that catalyst makers synthesize and then infuse with copper ions. The copper ions provide the active sites for the reduction reaction, and the zeolite’s small pores exclude hydrocarbons from the exhaust that could clog the system.
Gounder is focusing on understanding the copper ions. If he and his colleagues can determine the copper ion configurations within the catalyst that are the most reactive at low temperatures, it may be possible to design a material to contain more of those desired active sites, he says.
Another type of NOx after-treatment device avoids the urea tank, which is hard to fit on smaller vehicles. This type, called a lean NOx trap, takes advantage of the oxidizing conditions in a diesel engine that make it hard to chemically reduce NOx. When the engine is running lean—that is, with a low fuel-to-air ratio—the trap uses platinum nanoparticles to catalyze the conversion of nitric oxide to nitrogen dioxide, which quickly forms solid nitrates on barium oxide nanoparticles, also in the trap. During quick periods of running rich—when the fuel-to-air ratio in the engine is higher—the trap then chemically reduces NOx. During these periods, the platinum nanoparticles catalyze the reaction of hydrocarbons from injected bursts of fuel with NOx released from the trap to produce nitrogen and water. This action regenerates the trap.
Lean NOx traps have been used in some new diesel passenger cars in Europe and the U.S., including at least one of the Volkswagen models involved in the scandal, but the burst of fuel needed to regenerate the traps impacts engine fuel efficiency, so the traps have been less popular than SCR systems. More important, they are sensitive to poisoning by sulfur, a common impurity in diesel fuel. Some researchers have tried to make sulfur-resistant catalysts, says William S. Epling, a chemical engineer at the University of Houston. But if a catalyst absorbs nitrogen oxides, he adds, it will likely absorb sulfur oxides, too, and turn them into sulfates. And sulfates tend to be more stable than the nitrates that form from NOx. Increasing the engine temperature and adding a burst of fuel to the trap can remove the sulfates, again at a cost to the vehicle’s fuel economy.
Lean NOx traps could also help solve another cold-start problem with SCR systems: The onboard urea doesn’t decompose into ammonia below 200 °C. A lean NOx trap can be tuned to produce ammonia during the regeneration phase at low temperatures.
“Emissions catalysis is by far the most complicated type of catalysis I’ve ever encountered,” Gounder says. “Most industrial processes, like in a refinery, are going to be controlled to operate in a very narrow temperature and pressure window that’s been optimized to do the reaction.” But the catalysts in emissions systems have to work under a variety of temperatures, weather conditions, engine loads, and engine speeds for the lifetime of the vehicle—10 years or about 193,000 km. “It’s an engineering marvel that these things even work,” he says.
Gasoline engines catch up
Although diesel engines are inherently more fuel efficient than gasoline engines, a new engine technology is allowing gasoline engines to catch up. It’s called gasoline direct injection (GDI), and 43% of the passenger vehicles sold in the U.S. in 2014 have the technology—a share that’s expected to grow in coming years.

In a traditional gasoline engine, a premixed blend of fuel and air enters the cylinder. But a GDI engine operates like a diesel engine in that fuel is injected directly into the combustion chamber. The air in the cylinder cools as the fuel vaporizes, enabling greater compression before ignition and improving efficiency. But unlike the lean conditions of a diesel engine, most GDI engines inject enough fuel that little oxygen is left over after combustion. Standard catalytic converters effectively chemically reduce NOx to nitrogen in this low-oxygen environment, so GDI engines do not need the selective reducing power of an SCR system.
But they have other emissions challenges. GDI engines produce more particles than either traditional diesel engines or gasoline engines equipped with filters, especially ultrafine particles less than 0.1 μm in diameter. Particulate emissions are a combination of soot, ash, and volatile organic compounds adsorbed to the soot. Such emissions are associated with health problems such as aggravated heart and lung disease.
GDI engines currently meet particle emissions regulations in the U.S., but the cars’ ultrafine emissions soon may fail tests in Europe: U.S. regulations are based on the mass of particles, while Europe’s are based on number—and the permitted number per kilometer driven will drop in September 2017 as part of tightening European regulations. This may mean particle filters will be necessary for GDI engines to meet those standards, says Cary Henry, who leads a research consortium of automakers at Southwest Research Institute.
Diesel cars and trucks are commonly equipped with ceramic or metallic filters, which capture 85 to 95% of particles. But for GDI vehicles, it may not be as simple as putting a diesel filter on a gasoline engine. Subtle differences in particle size and composition between GDI and diesel engines may significantly impact the design of gasoline particulate filters for GDI engines, Henry says.
Soot is elemental carbon, and ash consists of metals such as zinc, calcium, and magnesium from gasoline additives and oil that accumulate in the engine. In a diesel particle filter, soot collects on the walls of the ceramic or metal filter. When the soot is oxidized or burned off to regenerate the filter, any accumulated ash also flakes off, Henry says. But GDI engines produce less soot than diesel engines, so instead of ash combining with the soot and flaking off, the ash could potentially, according to Henry, deposit on the walls, clog the filter, and cause back pressure that increases the load on the engine and reduces efficiency.
William F. Northrop, a mechanical engineer at the University of Minnesota, and his colleagues are testing filters and working to better understand particle emissions from lean-burn GDI engines. In diesel engines, filters keep particulate emissions to about one-tenth of the limit, Northrop says, and he thinks appropriately designed filters could do the same for GDI cars. “I really do think filters are going to come. It’s just a matter of when and how.”
History suggests he’s right. Although emissions control problems for NOx or particles seem difficult to conquer, so were the first attempts to control the belching emissions from tailpipes beginning in the 1980s. Since then, better engines and emissions controls have already cut emissions from heavy-duty trucks by at least 90% (Environ. Sci. Technol. 2009, DOI: 10.1021/es9008294). And they’ve managed to do it while also dramatically improving fuel economy and vehicle performance. Even amid the Volkswagen scandal, Jim Parks, a physicist at Oak Ridge National Laboratory, says the vast majority of diesel vehicles made since 2007 are clean and fuel efficient: “Clean diesel technology is not a myth.”

Melissae Fellet is a freelance writer. A version of this story first appeared in ACS Central Science: http://cenm.ag/noxemissions.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter