Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Reaction Mechanisms
Chemists announce the end of the innocence for cyclopentadienyl
Supposedly unreactive ligand caught in the act of protonation, suggesting new opportunities in catalyst design
by Stephen K. Ritter
June 10, 2016
| A version of this story appeared in
Volume 94, Issue 24

Sometimes chemists set themselves up for a surprise. Following sets of experiments in which something doesn’t happen and doesn’t seem likely to happen, they soon believe it never will. Until it does.
Two research groups have independently uncovered one of these surprises involving the popular transition-metal ligand pentamethylcyclopentadienyl, or Cp*.
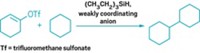
Chemists have traditionally thought of cyclopentadienyl ligands as being “innocent,” which means they offer electronic support to a metal catalyst but generally don’t do anything chemically. The two groups were studying reactions involving Cp*Rh(bipyridine), often used in hydrogenation reactions and in hydrogen-forming reactions, when they found that the expected metal hydride intermediate was followed by formation of an unexpected intermediate in which the hydrogen had migrated to one of the carbon atoms in the Cp* ring.
“These two reports showing that the seemingly innocent Cp* ligand can reversibly form a C–H bond by proton transfer from rhodium hydride are remarkable,” comments chemistry professor David Milstein of the Weizmann Institute of Science, who was not involved in the research. “Considering the ubiquity of cyclopentadienyl metal complexes in homogeneous catalysis, this pathway should be seriously considered in the design and understanding of reactions in which proton/hydride transfer may be involved.”
Alexander J. M. Miller of the University of North Carolina, Chapel Hill, who led one of the teams, says chemists had previously worked out mechanisms involving hydride intermediates that made sense and thought the story ended there. But they did not exercise due diligence and poke around enough to see that a protonated Cp* intermediate, denoted Cp*H, could be involved as well. “What’s more surprising,” Miller points out, “the Cp*H complex is not a dead end. This diene complex is still an active catalyst.”
Miller’s group came across the Cp*H intermediate while investigating hydride transfer reactions with the cellular enzyme cofactor nicotinamide adenine dinucleotide (NAD+) to form the reduced product NADH (Chem. Commun. 2016, DOI: 10.1039/c6cc00575f).
Meanwhile, a team led by Harry B. Gray and Jay R. Winkler at Caltech and James D. Blakemore at the University of Kansas discovered the Cp*H intermediate while investigating the coupling of protons to form H2 when treating Cp*Rh(bipyridine) with acid (Proc. Natl. Acad. Sci. USA 2016, DOI: 10.1073/pnas.1606018113).
“These discoveries illustrate the versatility of mechanisms by which protons and hydrides can be delivered to and from metals,” comments Morris Bullock, director of the Center for Molecular Electrocatalysis at Pacific Northwest National Laboratory. “While these examples are for rhodium, the prevalence of cyclopentadienyl ligands in organometallic catalysts raises the possibility that similar reactivity could be widespread and involve other metals, and may be intentionally exploited in the design of new catalysts.”
This article has been translated into Chinese and can be found here.
To see all of C&EN’s articles that have been translated into Chinese, visit http://cen.acs.org/cn.html.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter