Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
Updated law boosts EPA regulatory power over chemicals
Congress gives agency more authority to regulate health and environmental risks and demand toxicity data
by Cheryl Hogue
June 28, 2016
| A version of this story appeared in
Volume 94, Issue 27

Armed with new powers from Congress, the Environmental Protection Agency is readying for action on chemicals. EPA is training its regulatory sights on three long-used solvents that agency scientists determined may pose a serious risk to consumers’ health.
What’s unusual is that for the first time in more than a quarter of a century, EPA is poised to restrict chemicals that have long been on the market. And in a striking change from the past, the likelihood of a federal court knocking down these regulations is much lower.
EPA’s odds of successfully restricting or banning chemicals improved markedly in June. After years of wrangling, Congress passed legislation to overhaul the nation’s 40-year-old statute governing chemicals, the Toxic Substances Control Act (TSCA). President Barack Obama signed it into law on June 22, enacting the Frank R. Lautenberg Chemical Safety for the 21st Century Act. The law is named for the late Sen. Frank Lautenberg, a New Jersey Democrat who for decades championed legislation to improve the safety of chemicals and the chemical industry.
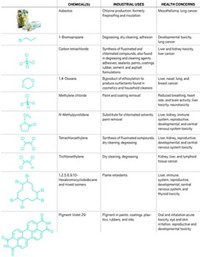
Three solvents, 20 flame retardants, and asbestos may be on the road to regulation
Substances at greatest risk for EPA regulation under the new chemical safety law rank among the dozens of substances and groups of compounds that the Obama Administration has already targeted for intense scrutiny. The agency has determined that some of these compounds—or some of their uses—pose a significant risk to human health or the environment and has announced plans to regulate them.

Three solvents used widely in paint and coating strippers—N-methylpyrrolidone, methylene chloride, and trichloroethylene—are likely to be among the first that EPA attempts to control under the new law. N-methylpyrrolidone and methylene chloride are suspected of causing health problems. Trichloroethylene is carcinogenic to humans, EPA says.

In addition, tris(2-chloroethyl) phosphate and 19 other could be the targets of EPA’s new authority to order chemical manufacturers to conduct toxicity testing on their products. The agency has said it needs these data to assess the substances and has asked companies to voluntarily produce the information. Many flame retardants are persistent and have been found in people’s blood.
Asbestos products, which EPA unsuccessfully tried to ban more than a quarter-century ago, may also come up for regulation. Since a court overturned that ban, lawsuits and other liability claims by those who developed cancer and other diseases from exposure to asbestos have driven many uses of the minerals off the market. That means few asbestos products remain for regulation, points out Richard Denison of the Environmental Defense Fund.
Additionally, the rewritten law directs EPA to put one group of substances near the top of its list for review and possible regulation: substances stored near drinking water supplies. Congress inserted this provision in response to contamination of the drinking water of 300,000 people in the Charleston, W.Va., area with commercial 4-methylcyclohexanemethanol, an obscure substance used to process coal. In that 2014 incident, West Virginia issued a days-long ban on the use of tap water—in large part because little is known about the human health and environmental effects of the material. Investigators found that the chemical leaked from a storage tank upstream of the intake for the water supply system.
In what is possibly the biggest change under the updated law, Congress has mandated that EPA systematically review the safety of commercial chemicals—defined as substances that aren’t regulated as food, drugs, or pesticides. The agency’s goal is to ensure that no chemical in U.S. commerce poses an unreasonable risk to human health or the environment.
Many health and environmental groups, product formulators, and chemical manufacturing companies are cheering this change. Activists anticipate that this new regulatory scrutiny will eliminate harmful substances from consumer products and protect people’s health. The chemical industry, meanwhile, hopes that EPA findings of no risk for specific compounds will rebuild consumers’ eroded faith in the safety of their products.
Although the safety reviews are likely to account for the biggest overall impact of the law, the Lautenberg Act makes another momentous change to TSCA. It unfetters EPA’s ability to regulate chemicals that are on the market.
The agency hasn’t even tried to regulate a commercial substance since 1991, when a federal appeals court slapped down the agency’s ban under TSCA of cancer-causing asbestos products. “The court effectively found that EPA had to prove it had analyzed every conceivable way of restricting asbestos and had chosen the one that was least burdensome to industry,” explains a statement from Safer Chemicals, Healthy Families, a coalition of health and environmental groups that lobbied for TSCA reform. The enormous legal onus of showing that it had chosen the least burdensome option effectively halted EPA from regulating chemicals currently in commerce.
Notwithstanding the court decision, EPA under the Obama Administration in 2009 began to scrutinize and plan for possible regulation of a handful of controversial chemicals or classes of substances, including bisphenol A and phthalates. The agency expanded this work in 2011 and now is working on 90 compounds or classes of chemicals. Those substances are linked to adverse reproductive or developmental effects; are persistent, bioaccumulative, and toxic substances; are probable or known carcinogens; are chemicals found in children’s products or consumer products; or are chemicals detected in human blood, urine, or tissue in biomonitoring programs. EPA has finished reviewing some of these compounds, determining that some pose no risk and flagging a handful for deeper examination or regulation.
In the modernized law, Congress directs EPA to build on the agency’s ongoing work. The agency must start by conducting mandatory risk reviews of 10 chemicals selected from the 90 it is already focused on and expanding those numbers in coming years.
The task of reviewing the risks posed by each chemical in commerce is huge and could drag on for a generation. Completing safety reviews of the estimated tens of thousands of chemicals now on the market is likely to take a couple of decades, observers from industry and environmental organizations agree. Plus, any restriction, ban, or phaseout of a particular chemical would take effect years after EPA completes a safety review of the substance, points out Melanie Benesh, a legislative attorney with the Environmental Working Group. Given the deadlines established in the law, Benesh figures it will take 35 years for the Lautenberg Act to fully affect chemicals on the market today.
In addition to requiring safety reviews and clearing the way for regulation, the updated law clearly empowers EPA to order chemical manufacturers to provide safety data about their products.
In contrast, the old statute put the agency in a bind. If EPA suspected that a chemical might pose a risk, the agency had to document that the substance actually presents a risk before it could require chemical makers to produce additional information. Plus, EPA could demand the information only by going through the formal federal regulation-making process. This takes a minimum of 18 months to complete but usually takes years. Now, EPA can issue an administrative order requiring manufacturers to provide toxicity data in addition to using the formal process.
Another change that Congress made in the law involves company trade secrets. Like the original TSCA, the revised statute requires EPA to shield from public view any data that chemical manufacturers claim as confidential business information. Health and environmental groups have complained—and many in industry agreed—that companies overused these assertions, which never expired under TSCA and didn’t require EPA review. Under pressure from the Obama EPA, chemical manufacturers have voluntarily lifted a multitude of outdated or questionable claims.
In a major change, the Lautenberg Act requires chemical makers to provide evidence to support a confidentiality claim when they make it. Claims will expire after a decade unless companies reassert and again substantiate them.
“We’re going to create a system that’s more transparent, that creates opportunities for information to be disclosed while appropriately protecting proprietary interests,” says Michael P. Walls, vice president of regulatory and technical affairs at the chemical industry group American Chemistry Council.
The rewritten law also directs EPA to review the thousands of trade secretclaims for chemicals’ identities that companies made in their TSCA filings over the past four decades. Companies seeking to maintain those claims must reassert and substantiate them, and EPA must determine whether they are still justified, says Richard Denison, lead senior scientist at the Environmental Defense Fund.
Up-front substantiation of confidentiality claims is one of several costs that small and midsized chemical manufacturers are worried about under the revised law, says Daniel Newton, senior manager of government relations at the Society of Chemical Manufacturers & Affiliates. EPA’s new powers to demand toxicity information will likely require greater outlays by companies too, Newton says. On top of this, Congress gave EPA the authority to impose fees on chemical makers and processors to offset some of the agency’s costs of implementing the various provisions of the Lautenberg Act. The agency will set those fees after consultation with industry, and the law specifies that small businesses will be charged less.
Overall, the amended law “is going to raise the cost of doing business,” Newton says.
But in the updated law, chemical makers also got a key provision that they sought as a way to rein in future regulatory costs. The Lautenberg Act makes it far more complicated for states to ban or regulate individual chemicals.
In the past decade, a patchwork of state laws and regulations grew because EPA didn’t—and couldn’t—act on chemicals of concern. Collectively, actions by states became a key motivator for the chemical industry to drop its long-standing, wholesale resistance to TSCA reform and begin lobbying Congress for changes.
Negotiators on Capitol Hill were long stuck on whether and how to override state action on chemicals. In a bipartisan compromise that led to final passage of the Lautenberg Act, lawmakers allowed states to retain bans and other chemical-related laws and regulations that were enacted by April 22 of this year. If EPA either determines a compound poses no unreasonable risk to human health or the environment or decides to regulate the substances, the law forbids states from acting on that chemical. States, however, may act on chemicals that EPA hasn’t reviewed or isn’t in the process of assessing.
Another key result of Congress’s revision of TSCA isn’t written into the Lautenberg Act. As EPA implements the statute, lawsuits challenging the agency’s moves are certain to crop up, attorneys for industry and environmental groups tell C&EN. Litigation asking courts to interpret the legal limits of a statute is par for the course when Congress gives new powers to an agency.
“Whatever can be challenged will be challenged,” Benesh says.
Less animal testing is a goal of the Lautenberg Act

The new U.S. chemical safety law aims to reduce the use of laboratory animals in toxicity tests.
It mandates that EPA opt, whenever practicable and scientifically justifiable, to rely on results of tests that are alternatives to chemical toxicity studies done with vertebrates.
“Those methods have to be scientifically reliable, relevant,” and capable of providing scientific information that is equivalent to—or better than—the data generated by long-used toxicity testing methods that rely on lab animals, says Michael P. Walls, a vice president at the American Chemistry Council, an industry group.
Alternatives are expected to include computational toxicity—a field that blends molecular biology, chemistry, and computer science—and high-throughput, cell-based assays that the pharmaceutical industry has long used. EPA has for years been exploring the use of data from these animal-free tests to support regulation of chemicals.
Animal welfare groups are strongly endorsing this part of the new law.
“Poisoning animals in chemical tests does not protect humans or the environment, plain and simple,” says Jessica Sandler, vice president for regulatory testing at People for the Ethical Treatment of Animals.
“By minimizing animal testing and focusing on the use of faster, cost-effective, and more reliable testing methods, private companies and the federal government can save lives, time, and money,” says Wayne Pacelle, chief executive officer of the Humane Society of the United States.
Results from new methods such as those involving computational toxicology may first be applied in efforts to prioritize chemicals for further scrutiny, Walls says. As these tests become more scientifically reliable, their results may be incorporated into risk assessment and regulatory decisions.
“At some point these approaches will no longer be alternatives but will be accepted practices for information gathering,” says the Society of Toxicology, a scientific and professional organization.
To date, EPA has not identified any methods to replace animal tests that it relies on for risk assessment and regulatory decisions.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter