Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Boron chemistry branches out
Electron poor but chemically rich element with a storied past continues to surprise with a surge of new developments
by Stephen K. Ritter
July 25, 2016
| A version of this story appeared in
Volume 94, Issue 30
When you mention boron to people and ask them what it brings to mind, there’s no shortage of answers. Some will think about borax (sodium borate), which is used to make glass and the playful concoction known as slime. Others may bring up boron fiber fishing rods.
Boron Sightings
This parade of boron- containing molecules represents a selection of the diverse set of compounds that researchers discussed at BORAM.
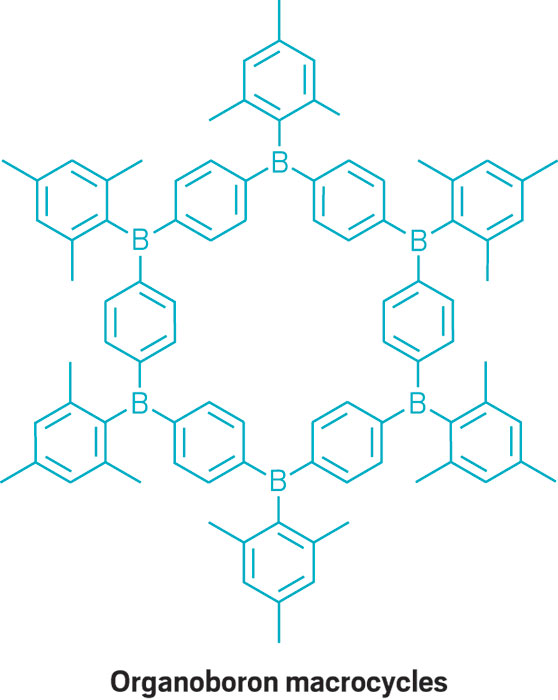
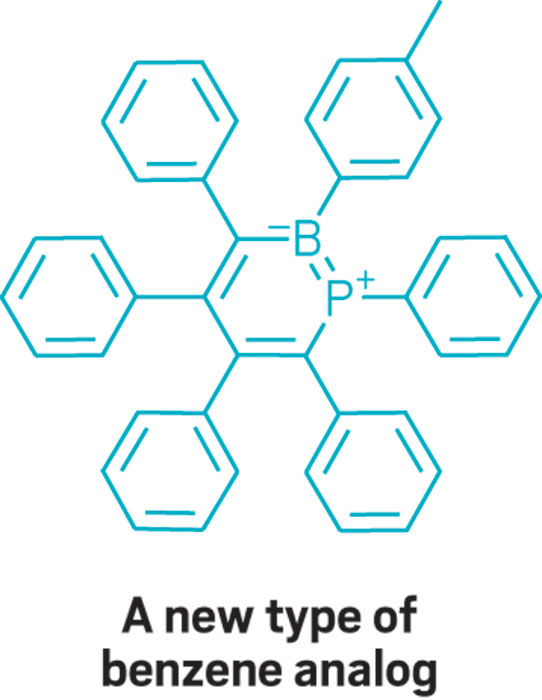
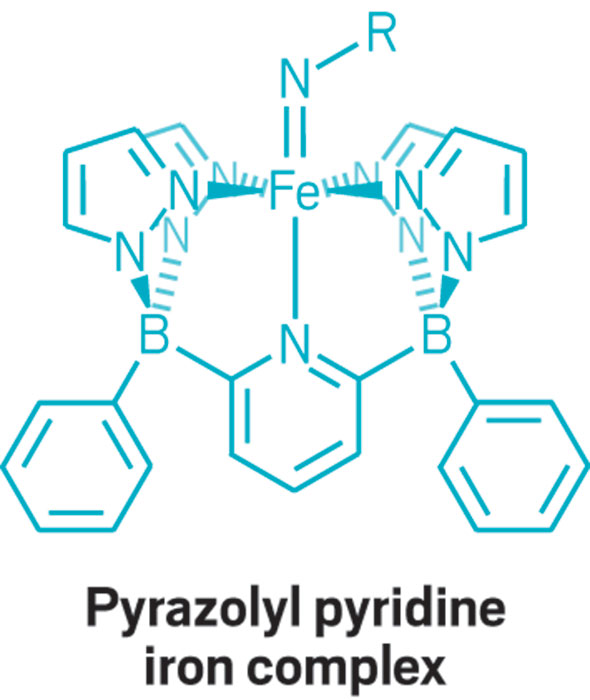
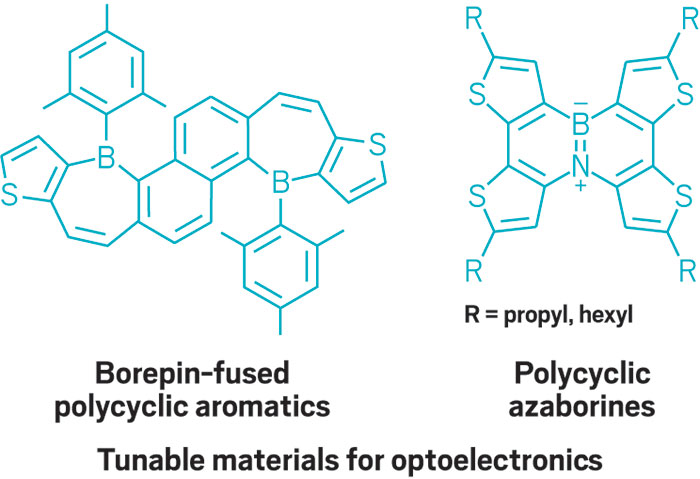
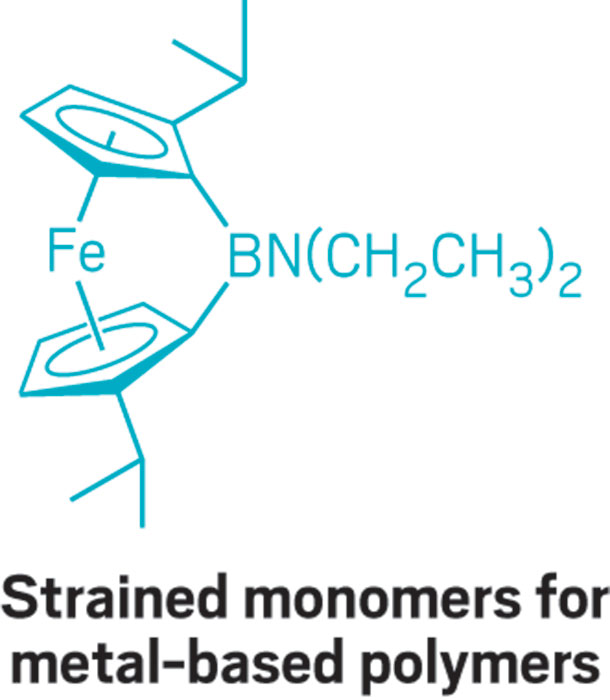
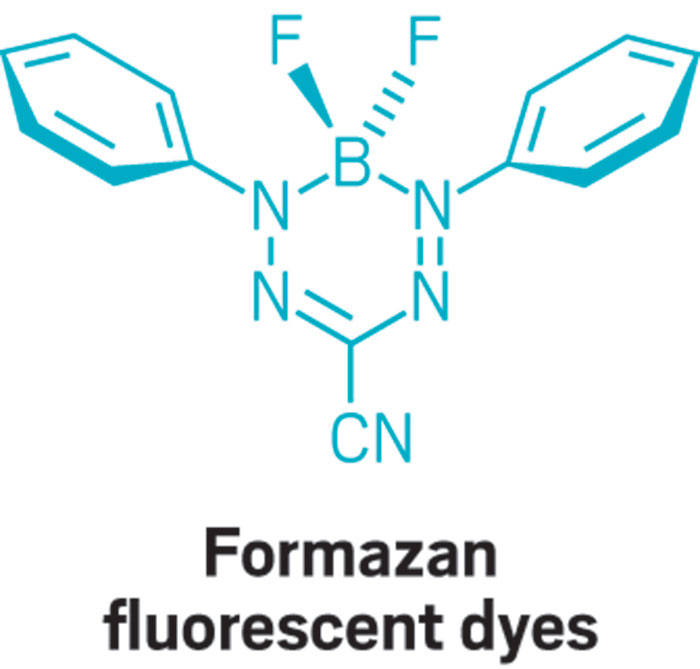
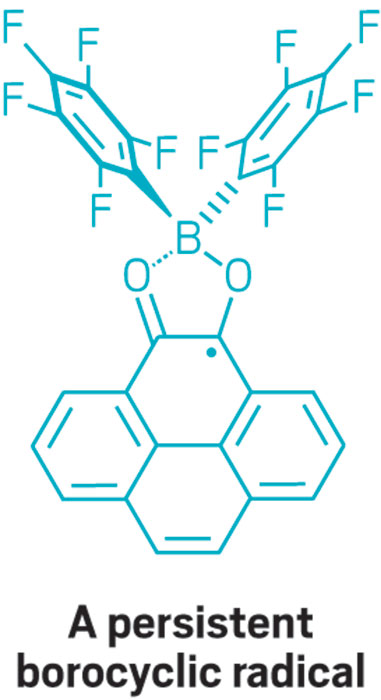
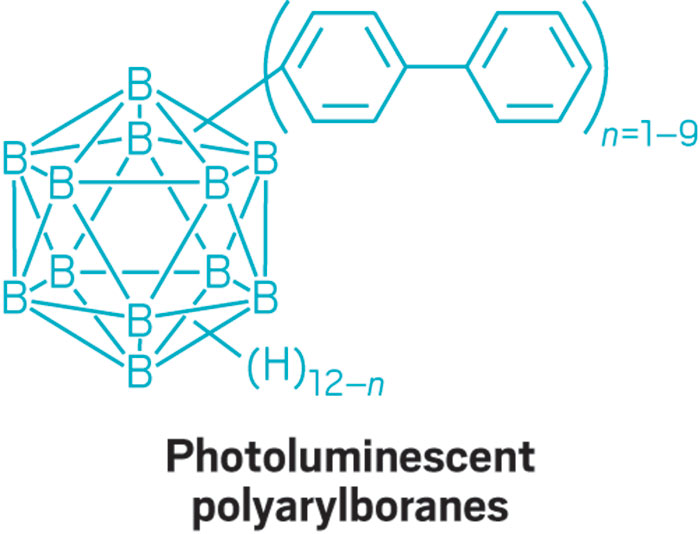
From chemists, you get the nerdy responses: Suzuki-Miyaura cross-coupling reactions, a beautiful green flame test, and funky bonding. Nearly everyone has a different answer to the boron question, and that diversity speaks volumes.
Boron is a quirky little element with four bonding orbitals but only three valence electrons. The odd number of electrons makes boron electron-poor yet rich with opportunities for chemical reactivity and influencing materials properties. Examples of boron’s prowess were on display last month at Boron in the Americas (BORAM), a biennial conference hosted this year by the chemistry department at Queen’s University in Kingston, Ontario.
The iconic element has a storied history to go along with its chemical diversity, according to Donald S. Matteson of Washington State University, who has contributed to the development of boronic and boronate esters since the 1950s. These compounds are now commonplace reagents that help transfer a reaction partner to the catalyst metal in cross-coupling reactions. At the boron conference, Matteson presented his research on developing silylated boronic esters for asymmetric synthesis.
“When we started out, the hot topic was polyhedral boranes,” Matteson told C&EN. German chemist Alfred Stock first characterized this series of boron-hydrogen cluster compounds in the early 1900s, developing glass vacuum line techniques for handling them. The chemical bonding of the boranes was later investigated by William N. Lipscomb, which led to his 1976 Nobel Prize in Chemistry.
Meanwhile Hermann I. Schlesinger and his student Herbert C. Brown developed the basic chemistry of the boron hydrides, such as sodium borohydride, a versatile reducing agent. Brown was awarded the 1979 Nobel Prize for his work, along with Georg Wittig for his related work on phosphorus. At the same time, Earl L. Muetterties and M. Frederick Hawthorne further developed polyhedral boranes and introduced carboranes.
Then boron esters became in vogue, Matteson added, and in the late 1970s, that led to Suzuki coupling reactions, which have had a big impact on medicinal chemistry and captured the Nobel Prize in 2005. In the past decade, Matteson observed, “the field of boron chemistry has become more international as boron’s use in medicine and its incorporation into new materials has taken off.”
“Boron chemistry is playing an increasingly central and unique role,” conference chair Suning Wang of Queen’s University told C&EN. Wang listed the element’s latest accomplishments: new cross-coupling reactions, metal-free catalysis, organic optoelectronic materials, pharmaceuticals, and imaging agents. Wang’s group, for example, is developing boron chelators that have photochromic properties when exposed to ultraviolet light and the potential to work as molecular switches. “Boron can do it all,” she said.
Frustration bears fruit
Boron chemistry’s growing diversity has helped spark a new kind of creativity among chemists, added Douglas W. Stephan of the University of Toronto, whose group has been making new discoveries in Lewis acid-base chemistry.
Lewis acids and bases are one of chemistry’s most cherished concepts. The central atom of an electron-deficient Lewis acid will readily share a spare pair of electrons donated from the central atom of an electron-rich Lewis base. The resulting bonding between acid and base forms a neutral adduct.
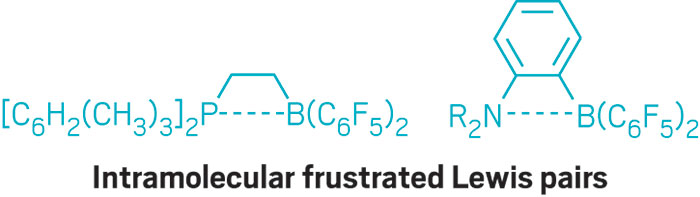
In 2006, Stephan and his coworkers took the concept to a new level when they devised a chemical construct known as a frustrated Lewis pair (FLP). The Stephan group recognized that when something comes between the pair, such as when the partners are dressed with bulky substituents or when they are separated by a spacer group, the pair is denied the ability to form a neutral adduct and becomes “frustrated.”
Lewis acids such as BF3 and Lewis bases such as amines are well-known industrial catalysts for their individual electron-accepting and electron-donating roles. But in FLPs, the unquenched pair creates an energetic mismatch between the empty acceptor orbital on the acid and the lone electron pair on the base. This situation gives the acid-base pair the ability to work cooperatively to bind and pull apart molecules, reminiscent of organometallic catalysts.
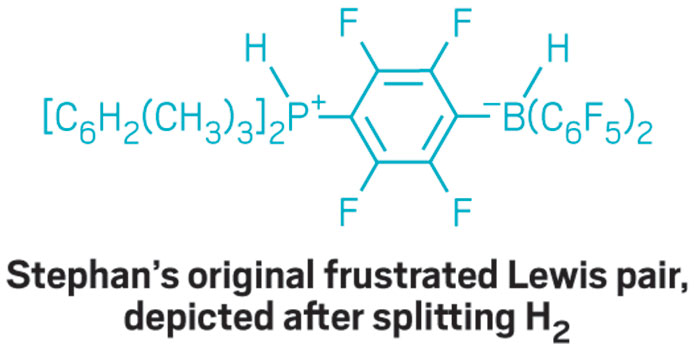
Stephan’s original FLP, a sterically encumbered intramolecular borane-phosphine pair, became the first non-transition-metal system known to reversibly bind and cleave H2, and it can do so at room temperature and pressure.
Stephan’s group, Gerhard Erker’s group of the University of Münster, and others have now created a variety of different types of boron-phosphorus FLPs, as well as FLPs combining boron and nitrogen or aluminum and phosphorus. The most remarkable consequence of FLPs so far, Stephan noted, has been their use in hydrogenating organic substrates, including imines, silyl ethers, polycyclic aromatic hydrocarbons, and olefins.
At BORAM, Stephan presented his group’s latest FLP work using B(C6F5)3 with a weakly basic solvent such as diethyl ether for catalytic hydrogenation of alkyl and aryl ketones to alcohols. Although the ketone reductions produce alcohols in high yields, graduate student Lauren E. Longobardi in the Stephan group reported that attempts to apply the strategy to aromatic diones unexpectedly result in formation of borocyclic radicals that are stable enough to be isolated. Early indications suggest these radicals could be useful catalysts in their own right.
Although the FLP focus has been on hydrogenation chemistry, researchers have also been using the reagents to activate CO, N2O, SO2, and other small molecules in reactions to functionalize organic molecules. According to Stephan, this collection of reactions shows that FLPs are “not a one-trick pony.”
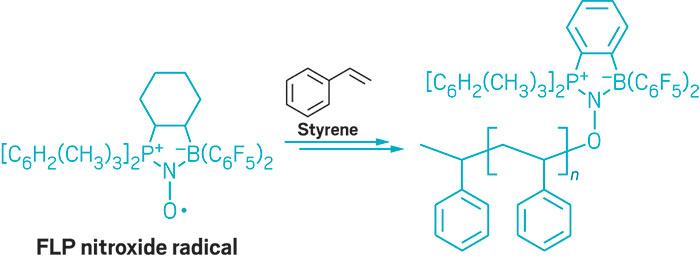
For example, when capturing NO, the University of Münster’s Erker and his colleagues found that FLPs form nitroxide radicals. These FLP-bound nitroxides facilitate selective oxidation reactions, such as forming O-substituted alkoxyamine derivatives of cyclohexadiene. They also mediate radical polymerization of alkenes such as styrene. And with the ability to reversibly trap CO2, N2O, and SO2, FLPs could provide a means of sequestering greenhouse gases, Erker added.
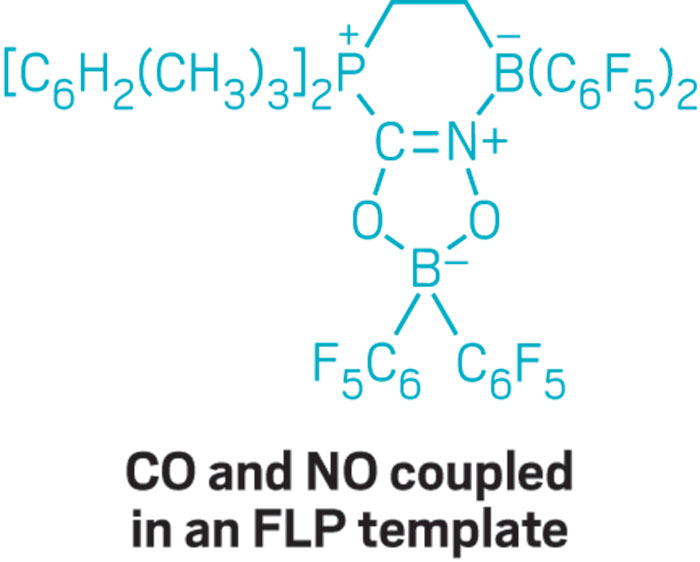
At the boron conference, Erker introduced the idea of adding a second borane to a phosphine-borane FLP, which results in a system where the two boranes compete for the phosphorus base’s attention. When CO and NO are combined, he explained, the FLP couples them together and incorporates them into the FLP framework via a radical process.
Both CO and NO are industrially important gases, Erker said, and both play essential roles in biochemistry. Yet their direct coupling in a metal-free environment had not been achieved before.
“Members of my group are always happy and smiling,” Erker said when showing a photo to acknowledge his coworkers. “That’s not just because they know they will eventually graduate and get their degrees, but because they are having so much fun working on this chemistry.”
Despite the early success with FLPs, they do have some limitations and downsides. Transition-metal catalysts still tend to be more active, provide better selectivity, and work on a greater variety of substrates for enantioselective hydrogenations. And although the reactions do eliminate the need for transition metals, which in some cases are in short supply and can be expensive, fluorinated arylborane Lewis acids are typically required, which are of concern because fluorinated compounds are persistent and bioaccumulate in the environment.
Stephan says some pharmaceutical companies are testing FLP chemistry, but to his knowledge it’s not yet being used commercially. Stephan isn’t sure how well the approach might work on a large scale, but using FLPs in continuous-flow microreactor systems might be a way to go. With time and new FLP designs, including using boron cations and boron dipyrromethene dyes to create photocatalysts, Stephan and Erker believe FLPs will only continue to get better.
Multiplying the bonds
As FLP chemistry shows, boron’s electron deficiency can be turned into a bonus. But it has also set up some road blocks, noted Holger Braunschweig of Julius Maximilian University Würzburg. For example, although diatomic multiple bonds of the main-group nonmetals carbon, nitrogen, oxygen, sulfur, and phosphorus are common, that’s not been the case with boron, Braunschweig pointed out.
Boron: The fifth element
During the Boron in the Americas conference last month at Queen’s University, in Kingston, Ontario, C&EN posed a question on Twitter: “What does boron as an element mean to you?” A few of the diverse responses are given below.
▸ Boron signifies electron-deficiency. Is that a hole in your valence shell, or are you just excited to see me?
Preston MacDougall (@ChemicalEyeGuy)
▸ Green flames. And I always liked organoboron chemistry.
Eva Horn Møller (@mediemedicin)
▸ One of the most important carbon-carbon bond-forming reactions you will find (Suzuki-Miyaura).
Adam Henwood (@AdamHenwood1)
▸ Funky bonding (Wade’s rules).
Stuart Cantrill (@stuartcantrill)
▸ Bortezomib, a boronic acid containing drug.
Inge Visscher (@visschei)
▸ Boomy rocket fuel.
Darth Osler (@autolycos)
▸ Glass beakers :)
Daniel Korenblum (@katchwreck)
▸ Tennis racquet strings. Didn’t know boron could be made into fibers before I got new ones (OK, this is physics really).
Len Fisher (@LenFisherScienc)
▸ Most versatile element in the PT.
Warren Piers (@wpiers1)
Boron prefers nonclassical bonding, Braunschweig explained, lending itself to exotic compounds such as polyhedral boranes with three or more atoms sharing electron pairs, rather than forming chains or rings with two-center two-electron bonds like most other elements do.
Braunschweig’s group has launched a dedicated effort to develop synthetic strategies to clear that hurdle, creating a host of new molecules with single, double, and triple boron-boron bonds. The team is further studying these species’ abilities to activate small molecules such as H2, CO, and unsaturated compounds.
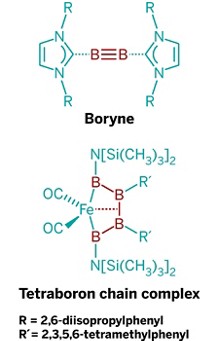
Among the successes so far, the Würzburg team reported an iron complex containing a four-boron chain, as well as a sandwich molecule containing aromatic triboracyclopropenyl rings (B32–). With these molecules, the researchers showed that boron can behave like carbon in alkanes and in rings such as benzene.
On the double bond front, Gregory H. Robinson of the University of Georgia prepared the first stable, neutral compound containing a B=B bond, called a diborene, in 2007. Braunschweig and coworkers have built on that discovery by synthesizing other diborenes and studying their reactivity. Then in 2012, the Braunschweig group prepared the first diboryne—a molecule with a B≤B bond.
Braunschweig noted that the development of electron-rich persistent cyclic diamino carbene and cyclic alkyl-amino carbene ligands has helped make this new chemistry possible. NHCs and CAACs, as these ligands are known, have unique σ-donating and π-accepting properties, effectively functioning more like transition metals to stabilize boron bonding, he explained.
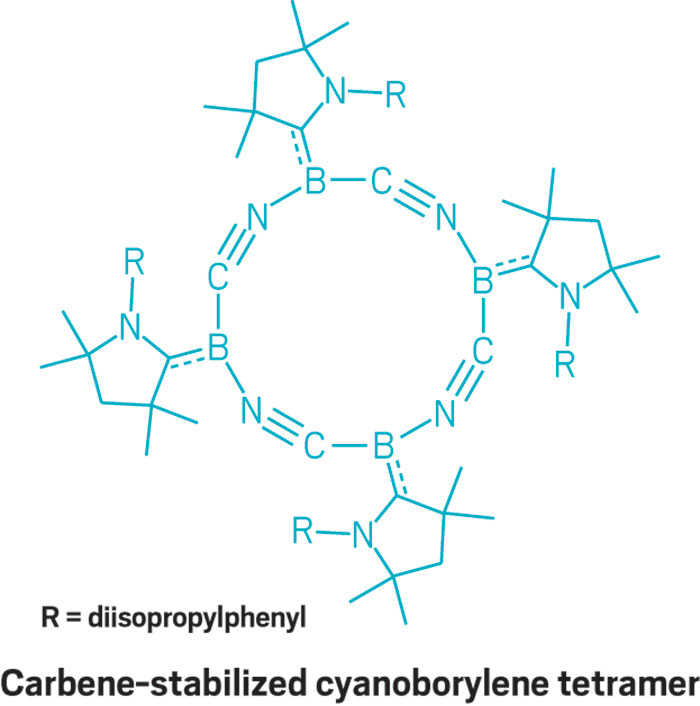
In a new example, Merle Arrowsmith in the Braunschweig group reported the synthesis of a collection of carbene-bound boron species. Some of the compounds are highly colored, “which makes them fun to work with,” she said. And the structures are fun to look at too. One of Arrowsmith’s unusual compounds is a cyclic cyanoborylene tetramer, whose 12-membered (BCN)4 ring structure remains stable in solution.
The Braunschweig team has also found that boron can act like a transition metal. For example, Marco Nutz unveiled chromium and molybdenum arylborylene complexes, which contain a reactive metal-boron double bond. These complexes are capable of transferring the arylborylene to compounds such as acetylenes, carbodiimides, and carbon monoxide, where boron donates electron density to carbon on its coupling partner.
Part of the reason for his group’s success, Braunschweig told C&EN, is that the researchers have made a conscious decision to explore the fundamental nature of chemistry without worrying about applications. “That has opened the door and put us on a pathway to unexpected results,” Braunschweig said.
Boron breaks into materials
Beyond catalysts and exotic new molecules, boron has also become a darling of materials science during the past decade. Boron is already known for its ability to add stability to glass, such as borosilicate Pyrex labware, and for amorphous boron spun into fibers and used as a structural material. Boron’s ability to tune the optical properties of materials is famous as well, as exhibited by the blue hue of the Hope Diamond.
At BORAM, scientists reported efforts to take advantage of boron’s electron deficiency to bolster the optical and electronic properties of new materials. Their approaches include strategically placing a few boron atoms in aromatic compounds and relying on boron’s ability to impersonate carbon to make graphene, nanotube, and fullerene analogs. These designed molecules can be used directly or polymerized and incorporated into thin films and layered materials for an array of applications, including detecting anions in environmental and biochemical settings, lighting up smartphone and television displays, and imaging cells and tissues.
Advertisement
For example, Shih-Yuan Liu of Boston College described the synthesis of new types of azaborines, which are aromatic compounds containing a B-N pair in place of a C-C pair. This B + N = 2C switch is becoming a common theme in modifying optoelectronic properties, Liu explained.
1,2-Azaborines, in which the B-N pair sits together in a ring, have been used in materials studies as substitutes for terphenylenes. But in looking to expand molecular options, Liu’s group has been working on synthesizing 1,4-azaborines, in which the boron and nitrogen sit opposite each other in a ring. One of the challenges chemists face with compounds such as azaborines is finding simple ways to make a variety of them for testing, he said. To get over that hurdle, Liu and his colleagues have now developed a general, three-step method to synthesize 1,4-azaborines from commercially available amines, dibromopropene, and dichloro(diisopropylamino)borane.
In another example, Shigehiro Yamaguchi of Nagoya University reported new synthetic strategies for incorporating boron into the framework of polycyclic aromatic compounds to create what he calls boron-doped nanographenes. Yamaguchi’s group has been testing these materials in lithium-ion battery electrodes.
On a different front, Yi Li of the Chinese Academy of Sciences’ Technical Institute of Physics & Chemistry described how the properties of fluorescent compounds such as triarylboranes are tunable by substituting different groups on boron. In one example, she explained how to design thermally sensitive triarylboranes for which a temperature change can trigger a fluorescence color change. Li showed how this system can be used to monitor temperature changes inside cells.
While those new compounds have only a few boron atoms, chemists are working on materials that contain 50% or more boron. At BORAM, Jingwen Guan of National Research Council Canada (NRCC), related that federal agency’s adventures in developing a commercial method to make boron nitride nanotubes (BNNTs).
BNNTs are structurally similar to carbon nanotubes (CNTs), Guan noted, but they have quite different physical properties. “Not surprisingly, these differences can be traced to the electron distribution,” he said. “CNTs are fully aromatic, but BNNTs exhibit local charge separation with a partially emptied orbital on boron.”
The result is that BNNTs are stronger, lighter, and more thermally stable than CNTs, Guan explained. Whereas CNTs decompose into CO2 at around 500 °C, BNNTs are stable to at least 900 °C without degradation and can stand open flames up to 2,000 °C for several minutes. “In fact, they are among the strongest, most stable materials known,” he said.
Another difference, Guan continued, is that unlike CNTs, which have metal-like conductivity and are black, BNNTs are insulators and white—they are transparent to visible light. Overall, BNNTs are optimal for automotive, space, and military applications, he said. One problem, however, is how to get ahold of a large amount of the material.
To that end, Guan and his NRCC colleagues developed a radiofrequency-guided thermal induction plasma system to prepare ton amounts of BNNTs from boron nitride powder. BNNTs are now commercially available, Guan told C&EN, and NRCC is licensing the technology for production and continues to work on their development. For example, at BORAM Guan described efforts to functionalize the nanotube surfaces by adding alkyl groups.
“Millions of tons of boron chemicals are now used annually in energy production, agriculture, and manufacturing,” commented David M. Schubert, principal scientist at U.S. Borax. “Yet we are still trying to understand borate chemistry—coordination chemistry, hydrogen bonding, and molecular architectures—to produce useful new materials.”
“Boron is an amazing element,” added Cathleen M. Crudden of Queen’s University, a conference co-organizer and a member of C&EN’s advisory board. Crudden’s group has been working on borenium cation catalysts, yet another class of new boron-based synthetic reagents. “The range of reactivity and types of molecules and materials where we find boron is truly fascinating.”
Boron's value chain
Versatile element is put to work in diverse chemical products and processes.
| Products | Markets | Estimated total global production by market |
|---|---|---|
| Refined borates (borax pentahydrate, boric acid, others) | Glass (electronic displays, fiberglass, specialized borosilicate glass), ceramics (tiles, tableware, porcelain enamel), oilfield, agriculture, other applications | Millions of metric tons |
| Specialty borates and borate esters | Wood preservatives, fire retardants, metallurgy, lubricants and industrial fluids, water treatment, personal care products, nuclear energy | Hundreds to tens of thousands of metric tons |
| Borohydrides | Pharmaceuticals, paper, electronics, energy | Metric tons to thousands of metric tons |
| Boron materials (BN, BC) | Aerospace, ceramics, electronics | Tens of thousands to hundreds of thousands of kilograms |
| Boronic acids and esters | Pharmaceuticals, electronics | Thousands to tens of thousands kilograms |
| Organoboranes | Pharmaceuticals, electronics, flavors & fragrances | Tens of thousands of kilograms |
| Amine boranes | Pharmaceuticals, surface modification, electronics, energy, polymers | Thousands to tens of thousands of kilograms |
| Polyhedral boranes | Electronics, propellants, energy, polymers | Up to hundreds of kilograms |



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter