Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Radiotracer helps neuroscientists study epigenetics in the brain
Molecule maps density of histone deacetylases in people
by Michael Torrice
August 17, 2016
| A version of this story appeared in
Volume 94, Issue 33
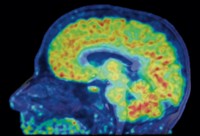
![Image of a human brain produced using positron emission tomography and the radiolabeled tracer [11C] martinostat. Image of a human brain produced using positron emission tomography and the radiolabeled tracer [11C] martinostat.](https://s7d1.scene7.com/is/image/CENODS/09433-notw4-wey1HRcxd-700?$responsive$&wid=700&qlt=90,0&resMode=sharp2)
Cells deploy an arsenal of enzymes to chemically modify DNA and its protein packaging. These so-called epigenetic modifications regulate the expression of genes, tuning the function of individual cells.
Neuroscientists have found that, in neurons, this enzymatic arsenal plays a significant role in learning and memory. And data suggest that dysfunction of epigenetic machinery is linked to neurological and psychiatric disorders, such as Alzheimer’s disease, schizophrenia, and depression.
Now a team of researchers at Massachusetts General Hospital led by Jacob M. Hooker reports a way to study one kind of epigenetic enzyme in living people’s brains using positron emission tomography, or PET (Sci. Transl. Med. 2016, DOI: 10.1126/scitranslmed.aaf7551).
“It’s a powerful new tool,” says Javier González-Maeso of Virginia Commonwealth University, who was not involved in the work.
The PET method maps the pattern of expression of epigenetic enzymes called histone deacetylases (HDACs), which pull acetyl groups off of proteins that package DNA in cells. “All of the studies on these enzymes so far have been in tissue culture or in animal models—in mice and rats,” González-Maeso says. “So this is the first study showing the expression of these epigenetic targets in humans.”
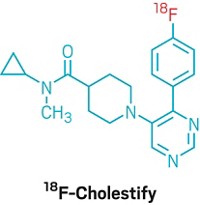
To measure the density of HDACs in the brain using PET, Hooker and his colleagues developed a radiolabeled molecule that can bind these enzymes. They tested hundreds of analogs of known HDAC inhibitors to pinpoint a molecule that could bind a specific class of HDACs, pass through the blood-brain barrier, and accurately quantify enzyme levels in the brains of rodents and nonhuman primates. The result was the molecule [11C]Martinostat.
In the new study, the researchers used the molecule to image the brains of eight healthy volunteers. They found that the pattern of HDAC expression was similar among the subjects.
The team also performed a preliminary study in cell culture to better understand the genes regulated by the HDACs that [11C]Martinostat inhibits. By identifying the genes controlled by these HDACs, Hooker says researchers studying patients with certain neurological diseases could use density maps produced by [11C] Martinostat to determine the genes whose regulation is disrupted in that particular disorder.
Hooker’s lab has already started imaging patients with schizophrenia, Huntington’s, and Alzheimer’s disease.
Besides determining how HDAC expression differs in disease states in the brain, González-Maeso also thinks the technique could monitor how the enzyme levels change over the course of a patient’s treatment to assess the efficacy of the therapy and to better understand its mechanism.

![Structure of [11C] martinostat. Structure of [11C] martinostat.](https://s7d1.scene7.com/is/image/CENODS/09433-notw4-LNP2-700?$responsive$&wid=300&qlt=90,0&resMode=sharp2)

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter