Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Nanodrug delivery
August 29, 2016
| A version of this story appeared in
Volume 94, Issue 34
July 11, page 29: The infographic about U.S. coin composition incorrectly stated that the penny acquired its current composition in 1984. It actually changed in 1982. Also, coins are struck from planchets (not cast from molten metal) and some (not all) dollar coins produced between 1849 and 1889 contained 90% gold.
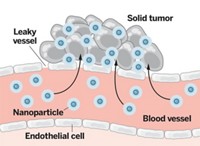
The article “Does Nanomedicine Have a Delivery Problem” (C&EN, June 20, page 16) nicely highlights a number of fundamental challenges that designers of drug nanocarriers must confront before these systems can be used reliably and safely in treating cancer. We suggest, however, that the formula used by Warren C. W. Chan of the University of Toronto and coworkers (Nat. Rev. Mater. 2016, DOI: 10.1038/natrevmats.2016.14), on which the C&EN article is based, provides a limited and perhaps overly pessimistic assessment of the nano approach.
Chan’s estimates of “delivery efficiency,” or the “fraction of injected dose that ends up in a tumor,” which cluster around median values less than 1%, are based on the following formula: delivery efficiency = AUC(tumor) * m(tumor) / T(end), with the three terms corresponding, respectively, to the area under the concentration curve of nanoparticles in the tumor, the mass of the tumor, and the last time at which data were taken. This formula calculates the average fraction of administered nanocarriers that are present in the tumor between the start of administration and T(end). The calculated value will depend critically on T(end), which could increase without bound without significant changes in the other two factors after nanocarrier deposition in the tumor is complete. Because T(end) depends on experimental design, delivery efficiency is not well-defined.
Any estimate of delivery of nanoparticles to a tumor, or any other target tissue, is by itself an incomplete figure of merit. The utility of nanocarriers depends on their ability to withhold release of the chemotherapeutic agent while circulating and then release the drug when located in the malignant tissue. Although strides have been made in this direction, nonspecific release of drugs into circulation, and off-target deposition of nanoparticles in tissues associated with toxicity, must also be taken into account. In some cases, the ability of nanocarriers to avoid absorption into tissues associated with toxicity may be a greater benefit than their ability to target drug delivery to tumors.
We concur with those quoted in the article who point out that the overall value (that is, improved therapeutic efficacy and reduced toxicity) of a nanocarrier system needs to be assessed relative to reference formulations of the same drug that do not use nanocarriers. The biodistribution of nanocarriers, the release kinetics of drugs from nanocarriers, and the pharmacokinetics of released drugs will all affect the success of a nanocarrier system.
Ronald A. Siegel and Jayanth Panyam
Departments of pharmaceutics and biomedical engineering, University of Minnesota
Minneapolis


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter