Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
14-year effort culminates in reaction that activates methylenes
Technique uses metal insertion to derivatize methylene C–H bonds catalytically and enantioselectively
by Stu Borman
September 5, 2016
| A version of this story appeared in
Volume 94, Issue 35

The ability to activate specific C–H bonds in organic compounds so they can be converted into C–C bonds or other derivatives has been a longtime goal in synthetic organic chemistry.
Over the past few decades, chemists have learned to activate several types of C–H bonds by inserting metal atoms into them. But the ability to activate the most common class of C–H bonds, those in methylene (CH2) groups, and to convert them into chiral centers has been a largely unsolved problem.
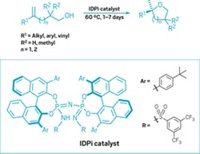
Jin-Quan Yu of Scripps Research Institute California and coworkers have now developed what could become a textbook reaction—the first that uses metal insertion to derivatize methylene C–H bonds catalytically and enantioselectively. It converts methylenes that are two carbon atoms away from amide and carboxyl groups into chiral centers (Science 2016, DOI: 10.1126/science.aaf4434).
Activating these so-called β-methylenes enantioselectively “was my first independent project, started in 2002, back in Cambridge University,” Yu says. “It took 14 years to finally achieve this goal.”
“Yu’s trailblazing group has managed to bring to reality what might have been considered seemingly impossible,” comments Erick Carreira, an expert on asymmetric synthesis at the Swiss Federal Institute of Technology (ETH), Zurich. “It represents a giant leap for the field that opens new vistas for the synthesis of optically active building blocks.”
Yu believes the approach can be extended to other classes of substrates besides those containing amides and carboxyls. A group at Bristol-Myers Squibb that he collaborates with is already using the new reaction to synthesize drug candidates.
Conjugate addition, a classical reaction that does not involve metal insertion, can also create chiral centers adjacent to carbonyl groups, but it requires that an alkene group be available or preinstalled at the β-position, and that is not always possible or easy to arrange.
In the new reaction, palladium and a simple bidentate ligand—one that coordinates with the substrate in two different ways—cause Pd to insert into one of the C–H bonds of a β-methylene. Chemists can then substitute aryl groups for the metal. The reactions have good yields and produce high ratios of products with specific chirality.
Although the ligand looks simple, engineering its structure so it worked just right took a considerable amount of effort and was key to developing the new reaction. Kendall N. Houk of the University of California, Los Angeles, and coworkers helped that effort by developing a computational model of ligand-substrate interactions.

This article has been translated into Chinese and can be found here.
To see all of C&EN’s articles that have been translated into Chinese, visit http://cen.acs.org/cn.html.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter