Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Chemists prepare an inorganic double-helix structure for the first time
The well-defined semiconducting material features intertwined tin iodide and phosphide chains
by Stephen K. Ritter
September 29, 2016
| A version of this story appeared in
Volume 94, Issue 39

Double-helix molecules are frequently encountered in biological and synthetic organic systems, where they typically provide improved strength and better electrical properties relative to materials containing linear chains or single helices. DNA is the defining example. A purely inorganic double helix has been hard to come by, until now.

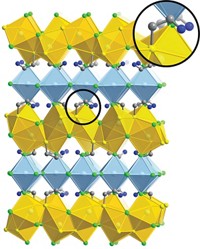
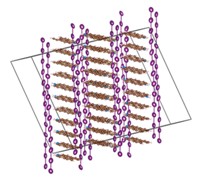
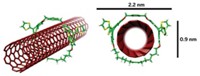
A team of some 20 researchers led by Tom Nilges of the Technical University of Munich has prepared the first completely inorganic substance, SnIP, featuring a well-defined double-helix structure. This semiconducting material consists of a twisted tin iodide (SnI+) chain intertwined with a twisted phosphide (P–) chain. The team prepared gram amounts of SnIP by heating tin, red phosphorus, and tin tetraiodide together (Adv. Mater. 2016, DOI: 10.1002/adma.201603135).
Chemists have been seeking out inorganic double helices for decades. Researchers have reported X-ray crystal structures of bulk LiP and LiAs containing spiral and coaxial chains, but it remained unclear as to whether they should be called double-helix structures. More recently, researchers have attempted making metal or metal salt double-helix materials using nanotubes or DNA as templates. But a nontemplated, carbon-free example with a fully characterized double-helix structure had remained elusive.
“This is a truly remarkable result,” says theoretical chemist Alexander I. Boldyrev of Utah State University. In 2012, Boldyrev’s group confirmed the controversial idea that inorganic double helices should exist by showing computationally that LiP can form a structure similar to DNA. “In the end, we might find that double-helix structures are not as rare in inorganic chemistry as we previously thought,” Boldyrev adds.
Nilges and his coworkers determined that the SnIP double helix is held together by weak interactions stemming from lone pairs of electrons on tin and phosphorus. And each double helix is coordinated to neighboring ones by interactions that are stronger than hydrogen bonding in DNA and impart hearty mechanical properties to bulk SnIP.
The team found that the needlelike SnIP crystals can bend in half without damage and that they can be cut down to form nanorods. With the extraordinary flexibility, coupled with its photoluminescence properties, “we are optimistic that SnIP can be used in semiconductor-based applications such as optical devices, including flexible solar cells,” Nilges says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter