Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Polyiodide chains
October 17, 2016
| A version of this story appeared in
Volume 94, Issue 41
The story “Polyiodide Chains Resolve Starch Mystery” (C&EN, July 11, page 9) refers to a very interesting article by Ram Seshadri and Fred Wudl of the University of California, Santa Barbara, and coworkers describing polyiodide chains in a pyrroloperylene-iodine complex (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201601585). The blue/purple color that forms when amylose (unbranched starch) reacts with iodine in solution has long been used as a starch indicator. The point of the new study is to show that iodine in such complexes can form extended polyiodide chains.
The Raman spectrum reported for the pyrroloperylene system in the crystalline state is similar to that previously reported for amylose-iodine, indicating that they have a similar structure. In describing the starch-iodine complex, the C&EN article states, “The helical iodide chain that forms wraps around starch molecules (amylose) to create a complex that produces a deep purple color.” I am concerned that this description of the starch-iodine complex is misleading, and I do not want it to add confusion to the historical picture.
The amylose-iodine complex was characterized crystallographically in the 1940s by R. E. Rundle and Frank C. Edwards as helical amylose with iodine atom chains inside the tubular helix (J. Am. Chem. Soc. 1943, DOI: 10.1021/ja01251a055). Complexes of iodine with α-cyclodextrin were later reported to have a similar structure.
I have more than a passing interest in this topic, as my thesis adviser and I studied the kinetics of formation of the aqueous amylose-iodine complex in the early 1970s and proposed a mechanism involving the formation of a relatively stable I113– segment followed by a rapid filling of amylose helical segments with iodide. Seshadri, Wudl, and coworkers show that stacks of pyrroloperylene molecules are interspersed with polyiodide chains of shallow helicity. But it seems evident to me that, in amylose-iodine, the polyiodide chains are inside the helix, not wrapped around amylose.
John C. Thompson
Coatesville, Pa.
Corrections
Sept. 5, page 2: A letter to the editor incorrectly stated when Lord Cherwell served as an adviser to Winston Churchill. Lord Cherwell advised Churchill during World War II, not World War I.
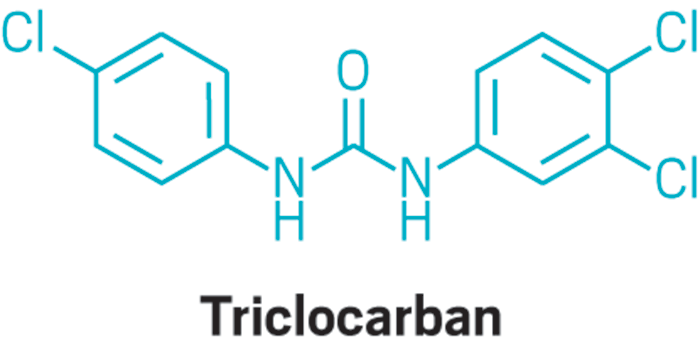
Sept. 12, page 16: Because of a production error, a structural diagram of triclocarban was cut off to incorrectly show two chlorine atoms as carbon atoms. Here is the correct structure.
Sept. 12, page 27: A feature story profiling Dow Chemical’s Peter Trefonas incorrectly identified when Dow acquired Rohm and Haas. The acquisition occurred in 2009, not 2001.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter