Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Porous fluoropolymer separates water-soluble organics
Nanoporous material is the first to selectively pull dyes and other compounds from water based on their charge and size
by Stephen K. Ritter
November 21, 2016
| A version of this story appeared in
Volume 94, Issue 46
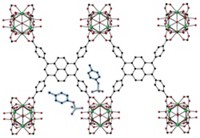
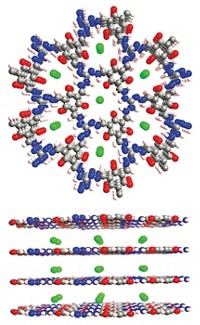
Porous materials such as zeolites, metal organic frameworks, and nanocarbons are known for their ability to selectively interact with and separate chemical species having similar sizes and functional groups. But the ability to separate charged molecules of various sizes, especially when dissolved in water, has remained a challenge. A team led by Cafer T. Yavuz of Korea Advanced Institute of Science & Technology (KAIST) has now reported a microporous network fluoropolymer that can selectively separate cationic dyes and other charged molecules from mixtures of water-soluble organics (Nat. Commun. 2016, DOI: 10.1038/ncomms13377). The researchers first prepared an inexpensive new covalent organic polymer, dubbed COP-99, by treating commercially available tetrafluorohydroquinone with potassium carbonate (shown). They found that the material pulls modestly sized charged molecules such as the dye methylene blue out of water, but it isn’t capable of sequestering larger dye molecules such as rhodamine B or uncharged molecules such as bisphenol A. The key to the material’s selectivity is its restrictive pore size and the exposed fluorine atoms, Yavuz notes, which both create hydrophobic pores and provide strong electronegative forces attractive only to charged organics. The KAIST team envisions a range of applications, including water treatment to remove artificial dyes, pesticides, and prescription drugs.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter