Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Roche launches network for cancer immunotherapy
Drug firm will spend $100 million at 21 institutions across nine countries
by Ann M. Thayer
November 17, 2016
| A version of this story appeared in
Volume 94, Issue 46

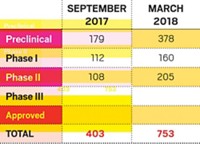
To support its position in the competitive immuno-oncology (I-O) field, Roche will invest up to $100 million in academic research. The drugmaker has created what it calls the immunotherapy Centers of Research Excellence, or imCORE, a network spanning 21 as-yet-undisclosed institutions across nine countries.
“The goal of imCORE is to facilitate access to new technologies and emerging data among the top researchers around the world,” Roche Chief Medical Officer Sandra Horning said when announcing the network. Roche anticipates its investment will help speed up preclinical and clinical research to advance new therapies that harness the immune system to fight cancer.
The Swiss drug company isn’t the first to try this approach. In May 2012, Bristol-Myers Squibb created the International Immuno-Oncology Network, which now includes 13 cancer research centers in the U.S. and Europe. In 2015, Bristol-Myers also set up a multi-institutional Immuno-Oncology Rare Population Malignancy program in the U.S.
Bristol-Myers’s Opdivo and Merck & Co.’s Keytruda are immune checkpoint inhibitors already enjoying success as anti-PD-1 treatments effective for certain patients. And Roche recently beat out AstraZeneca and Pfizer by getting approval for the first PD-L1 inhibitor.
Checkpoint inhibitors are expected to account for 95% of the cancer immunotherapy market by 2025, says Rachel Webster, a senior director at the market research firm Decision Resources. Combined sales will grow from about $5 billion this year to nearly $60 billion a decade from now.
But a problem for the large drug companies dominating the increasingly crowded space is that “they all have similarities in their pipelines,” Webster says. “Everybody is asking, ‘What is next?’ And collaborating with academia is perhaps one of the most efficient ways of being able to identify novel targets.”
Companies are finding that a promising therapeutic approach is to mix I-O drugs with agents that have different mechanisms of action. Webster suggests that academic researchers can help provide the scientific rationales for doing so while assisting in finding biomarkers that signal which patients will respond.
Correction: This story was updated on Dec. 5 to correctly reflect Decision Resources’ expectations about checkpoint inhibitors.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter