Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Renewables
Will the artificial leaf sprout to combat climate change?
Technology to produce fuels from sunlight, water, and carbon dioxide faces scientific and economic challenges
by Katherine Bourzac, special to C&EN
November 21, 2016
| A version of this story appeared in
Volume 94, Issue 46

On a gray and rainy October day in Palo Alto, Calif., the palms lining the driveway to Stanford University silently drip and the perfectly manicured lawns are lush and green. For a group of chemistry researchers a few buildings away, these plants are not merely a spot of beauty on the way to the office, but a daily inspiration.
In brief
Renewable sources of electricity will help us reduce climate-changing carbon dioxide emissions, but our society will also need renewable fuels. To find a renewable way to produce such fuels, some scientists have developed technologies that use sunlight to split water to make molecular hydrogen or reduce CO2 into hydrocarbons. These technologies face significant engineering challenges to produce fuels efficiently and economically.
Inside Thomas Jaramillo’s Stanford lab, these researchers are trying to catch sunlight in a bottle—a pair of connected bottles, actually. The glassware is filled with water and flooded with light from a solar lamp. Inside one bottle is a shiny iridium wire. In the other is a tiny electrode coated with an experimental catalyst. This system uses light to drive plant-inspired water-splitting reactions, generating molecular hydrogen. Behind an adjacent glass cabinet, another experiment is under way with a mess of plastic tubing ferrying away products made, in a plantlike manner, in another reactor fueled with carbon dioxide.
Jaramillo’s lab is one of many working on artificial photosynthesis. These scientists hope to do what plants such as palms and grasses do: use solar energy to power the transformation of abundant chemicals such as water and CO2 into fuels and other useful chemicals.
The need is dire. For the first time in human history, levels of planet-warming CO2, much of which has been produced by burning fossil fuels, reached a global average of 400 parts per million in 2015. Climate scientists have been eyeing this symbolic level with dread. The last time CO2 levels reached 400 ppm was a few million years ago, before our species emerged. Many scientists are looking for ways to slow and possibly reverse this trend.
“Our goal is to close the carbon fuel cycle,” says Harry Atwater, director of the Joint Center for Artificial Photosynthesis (JCAP), a Department of Energy project housed at California Institute of Technology. CO2 released when fuels are burned would be captured and made into new fuels. “It’s an audacious concept,” he admits.
Other scientists in the field are even more audacious, dreaming of transitioning to a chemical industry that captures CO2 as a feedstock. Instead of relying on petrochemicals, industry would use CO2 to make all kinds of hydrocarbons, not just fuels but valuable chemicals, effectively sequestering CO2 in polymers, drugs, and other materials. After all, these scientists say, plants make complex carbohydrates and other chemicals using the same starting materials. Still other researchers dream of displacing fossil fuels as a source of molecular hydrogen and of eventually enabling a transportation and shipping system rebuilt around this unconventional fuel.
In the past five years, chemists say, solid demonstrations of such systems, often called artificial leaves, have shown that what once seemed like a dream is possible. They’ve created catalysts and reactors for converting sunlight, water, and CO2 into hydrogen gas, formate, isopropyl alcohol, plastic feedstocks, and other fuels and chemicals. Some systems convert as much as 10% of the energy in absorbed sunlight into chemical bonds—far outperforming natural photosynthesis, which achieves only 1 or 2% efficiency.
“We’re telling people this concept is working with decent efficiency,” says Peidong Yang, a chemist at the University of California, Berkeley. Proving that it can work at a competitive price will be more challenging. Researchers will have to show that the artificial leaf can compete with more established technologies that make hydrogen and fix carbon using electricity, rather than sunlight. Those that produce hydrogen have made it to the market on a small scale. The artificial leaf may be more cost-effective on large scales, but to get there, it will have to first compete with existing systems. Researchers must show that artificial leaf technology works outside the lab, with not just decent, but salable, efficiency—and that they can make a fuel or chemical that fits an unmet need in the market, with costs that are competitive.
Green dreams
The human health and environmental costs associated with fossil fuels are what drive researchers working on renewable energy technologies such as artificial leaves. “Fossil fuels are easy to pick on, but they’ve allowed us to have the quality of life we do,” Jaramillo says. Transportation, plastics, the computing industry, food in abundance—most of the comforts of the developed world depend on fossil fuels, and a growing number of people in developing countries are asking for access to the same good things.

Adoption of renewable electricity is growing, but it won’t be enough. There is a gap in the clean energy picture. For example, an estimated 40% of our transportation and shipping infrastructure cannot be electrified, says Nathan Lewis, a chemist at Caltech. Today’s batteries don’t store enough energy by weight—especially in the case of planes. Batteries are too heavy. “There will never be a battery-powered 787,” he says. Fuels’ ability to store tremendous amounts of energy in chemical bonds just can’t be beat: The best batteries today store 200 watt-hours per kilogram; gasoline’s chemical bonds store 12,000.
To meet the growing demand for energy and chemicals without further exacerbating climate woes, we need new technologies, artificial leaf developers say. “Our lab and this field are aimed at new ways of creating the same types of fuels and chemicals, in a way that’s renewable,” Jaramillo says.
So Jaramillo and others are trying to mimic the chemistry inside leaves. Natural photosynthesis starts when chlorophyll and other pigments absorb light. The energy in photons excites electrons that plant enzymes then use to split water. This process generates oxygen gas, which is released, along with more electrons and hydrogen ions that are used in other reactions, including fixing CO2.
Whatever the ultimate product they have in mind, chemists working on artificial photosynthesis also start with light absorption and water splitting. In these artificial leaves, inorganic semiconductors absorb the light and deliver excited electrons to catalysts that then do the water-splitting reaction. Sometimes the light absorber and the catalyst are one and the same material.
In 1972, two Japanese researchers, Akira Fujishima and Kenichi Honda, showed that electrodes made from the right materials could split water when illuminated. On one side of their system, a titanium dioxide electrode catalyzed the oxygen-producing part of the reaction; on the other side, platinum catalyzed the production of molecular hydrogen. The electrodes in this early system were both expensive, and the efficiency of using the energy from photons to split water was just 0.1%.
Researchers have been building on this advance ever since, often calling it by the less whimsical name of photoelectrocatalysis. One challenge has been to lower the cost of the materials, especially the catalysts: Platinum and iridium, the most efficient catalysts, are too expensive to do artificial photosynthesis at large scales. Also, researchers must develop materials that are stable in acid and base over a long time period. To facilitate the movement of electrons and hydrogen ions, these reactions have to be done in either an acidic or basic solution. That means both catalysts need to work at the same pH or they must be separated by expensive, specialized membranes.
In 2010, artificial photosynthesis got a big boost when the Department of Energy launched JCAP. In its first five years, the center focused on the water-splitting problem, with $116.2 million in funding.
Among other things, the center funded and conducted research on high-throughput experimental screening and computational discovery of catalysts. Researchers also worked on ways of encapsulating photoelectrochemical electrodes so that they could withstand the wet, basic or acidic conditions in these reactors. And they worked on systems-level reactor designs.
The result was a prototype that looks like a glass box holding shiny venetian blinds. This integrated system uses light to split water with an efficiency of 10% and can run continuously for hundreds of hours. In this device, an electrode made of a semiconductor absorbs sunlight and the resulting electrons and holes then pass to two nickel catalysts that split water. The whole thing is submerged in a slightly basic solution. The electrodes are coated with a thin film of titanium dioxide to protect them from wear.
Atwater says the device was a milestone, and it shows that the science of solar water splitting has matured into a technology that needs further development by applied scientists and engineers. In October, DOE officially announced that research on the prototype has been transferred from JCAP, which is a basic science center, to an applied division of DOE, the Fuel Cell Technologies Office.
Hydrogen produced by a device such as JCAP’s could be used in energy-generating fuel cells, whose emissions are just clean water. There’s not much of a market for running vehicles with fuel cells yet, but government programs in Japan, Germany, the U.S., and elsewhere are pushing for it. Toyota and Hyundai make hydrogen fuel cell cars; Honda has announced plans to make one, too.
Or this renewable hydrogen could go into the broader market. Each year, more than 50 metric megatons of hydrogen are made by nonrenewable steam reforming of methane. There is a large existing demand for hydrogen today—but mostly not for use as fuel. Hydrogen is primarily used in petroleum refining and fertilizer production—CO2-emitting processes themselves. A renewable source of hydrogen, though, would make these processes a bit greener.
The Fuel Cell Technologies Office’s target price for hydrogen is $4.00 per kilogram after transportation, storage, and distribution. A kilogram of hydrogen stores about the same amount of energy as a gallon of gasoline. Because artificial photosynthesis is a newer technology, DOE’s cost targets for hydrogen produced by those devices are not as aggressive. The goal is to reach $7.00 per kilogram produced by artificial leaves by 2020, says Sunita Satyapal, director of the Fuel Cell Technologies Office.
Clean carbon
Meanwhile, JCAP and others have moved on to a harder problem. The developed world has already invested in a fossil fuel infrastructure—not one based on hydrogen. The ideal end point for artificial photosynthesis, then, would be a renewable version of the chemicals we already use, such as gasoline and polymers. To get there, chemists have to figure out how to do more of what plants do: use the electrons and hydrogen produced by water splitting to fix CO2.
“The most challenging thing about CO2 is it’s such a stable molecule,” says Karthish Manthiram, an incoming professor at Massachusetts Institute of Technology who works on CO2-reducing catalysts. “From a catalysis standpoint, it’s about trying to massage the thermodynamics.”
In terms of thermodynamics, says Aleksandra Vojvodic, a computational chemist at the University of Pennsylvania, making molecular hydrogen is a relatively simple process—the reaction requires just two electrons. But reducing CO2 is a significant step up in terms of complexity. Researchers must come up with catalysts that can juggle eight electrons. Linking together multiple carbons in long chains is even harder.
“The science of CO2 reduction is very difficult,” Atwater says. “We would be very satisfied if we could understand the factors that allow one to make one fuel or another, and make something useful.” But the specificity of these reactions tends to be low. So far, catalysts can produce single-carbon molecules, and they tend to produce a mishmash of them that must be separated from each other.
Until recently, making anything besides hydrogen fuel using a catalytic process seemed like a futuristic prospect, Manthiram says. “A couple years ago, I would have said we’re decades away,” he says. “It feels imminent now.” That’s because just about every week, you can find new record-breaking CO2 reduction catalysts being described in scientific journals.
Photoelectrochemical CO2 reactors are similar in design to water-splitting ones, with electrodes and catalysts submerged in an electrolyte. The major difference is that concentrated CO2 must be bubbled into the electrolyte. The cost of purifying and concentrating the gas must be factored into economic analyses of the technology. One source of concentrated CO2 for an artificial photosynthesis reactor could be a carbon capture system at a coal-fired power plant.
The best photoelectrocatalysts so far are a trio of semiconductors, says Emily Carter, a computational chemist at Princeton University. Gallium phosphide, copper indium sulfide, and cadmium telluride can all catalyze the reduction of CO2 into methanol, which could be used to make gasoline.

“This sounds great, except you’re producing such small quantities of methanol,” Carter says. That small amount of methanol must then be separated from the solution, which takes a lot of energy. Carter has been working on these methanol-generating reactions for years. She says they’re still not very well understood. Researchers don’t know why, but all of these catalysts work only in acidified water, with pyridine in the mix. Her recent research suggests that the pyridine acts as an intermediary in the reaction.
Advertisement
“It’s the philosophy of chemists: If you can understand it, maybe you can make it better,” Carter says. Better in this case would probably mean not just making methanol at higher concentrations, but making some other hydrocarbon that would naturally separate from water.
Carter says she knows she’s asking a lot of these photoelectrocatalysts. They must not only do a good job catalyzing the reaction, but they also have to be good light absorbers and good at separating and moving charges. “I’m coming to the conclusion that it may be easier to optimize a few properties, not all of them,” she says.
Dark reactions
Besides developing more efficient systems to produce hydrogen and hydrocarbons, the artificial leaf field will have to overcome a significant hurdle to thrive in the market. There are already more mature technologies for making hydrogen by water splitting, and there are analogous technologies under development that use CO2 as a feedstock. But they do so in the dark. These devices use electricity, sometimes from a solar cell or some other renewable source, to generate hydrogen or a hydrocarbon.
In fact, says DOE’s Satyapal, “photoelectrochemical production of hydrogen is at the lowest technology readiness level” of all the renewable methods DOE is considering. Electrocatalytic systems that skip the sunlight and use electricity and platinum-group metal catalysts to split water and make hydrogen are already on the market and are much more developed, she says.
There’s a good chance the artificial leaf might wither on the vine because of tough competition from existing technologies, says Katherine (Kathy) Ayers, vice president of R&D at Proton OnSite and a member of JCAP’s scientific advisory board. The Wallingford, Conn.-based company makes electrochemical hydrogen generators. Electrolysis systems that use expensive catalysts and electricity from a wall plug already meet the demand for distributed, small-scale hydrogen production for use in fuel cells and other applications, including gas chromatographs and weather balloons.
In theory, photoelectrocatalytic systems that use less expensive catalysts, such as JCAP’s nickel-based ones, offer lower costs if they’re operated on large scales. An analysis done by the center suggests that an economically feasible plant could produce 610 metric tons of hydrogen per day. All that hydrogen would store 1 gigawatt of energy.
But getting these kinds of systems to market will require passing through a phase in which they’re operating at smaller scales, and this would put them in direct competition with established electrocatalytic systems, Ayers says. At smaller scales, costs such as those for labor or electrical and water handling systems dominate. And some of these costs are likely to be higher for photoelectrocatalysis at first, simply because it’s new. Electrocatalysis companies have been around for decades. The artificial photosynthesis systems will also likely integrate new materials, which are usually more expensive to manufacture and involve greater complexity because they have to handle light, whereas electrolysis proceeds in the dark.
“If photoelectrocatalysis is not cost-effective until the megawatt scale, how do you make that leap?” Ayers asks.
A similar market challenge may exist for technologies involving photoelectrocatalytic reduction of CO2, although these systems, and their dark electrocatalytic competition, are not as mature. Today, the best electrocatalyst to do the job is copper, which reduces CO2 to methane. Methane gas bubbles out of solution—a clear advantage over methanol produced in some photoelectrocatalytic systems. Chemists’ gripe with copper and other electrocatalysts is that they reduce CO2 too slowly to be industrially viable. “It’s not just too slow, but too slow by a factor of a million or a billion,” Jaramillo says.
Still, a few start-ups are betting on the process. Opus 12 of Berkeley, Calif., for example, is working on scaling up a dark electrocatalytic process for reducing CO2 to make polymer building blocks and other chemicals.
If dark processes are further along, why bother with photoelectrocatalysis in the first place? Carter says there are good reasons, if you care about using renewable energy. Electrocatalysis will ultimately be limited by the efficiency of the electricity source. If the energy is supplied by a solar cell that converts 20% of the energy in sunlight into electricity, then the upper efficiency limit will be 20%—and it will reach that only if everything else is perfect. If the electrocatalyst of that system then has an efficiency of 10%, the overall efficiency will be only 2%. Plus, there will be loss at every connection point. Photoelectrocatalysts may offer better efficiency overall because they wouldn’t have this multiplication problem, Carter says.
Living proof
To get around the problem of efficiently making more valuable chemicals at higher purity, some researchers are turning back to the original inspiration: biology. Harvard University synthetic biologist Pamela Silver doesn’t think it’s necessary for chemists to twist themselves into knots trying to mimic biology. The question for her is how to use what biology has already given us. “Plants are the best chemists there are,” she says. “Trying to supplant that with pure chemistry” may be making things unnecessarily difficult. “How is this going to work if we don’t use biology?” she asks.
Silver’s group is one of a handful working on combining synthetic catalysts that make hydrogen with living microbes that feed on it to fix CO2 and make complex carbon compounds. So far, the photosynthetic systems that can make the most complex hydrocarbons using sunlight (besides plants themselves) are these bionic ones.
She started working on this in 2013, when chemist Daniel Nocera moved his lab from MIT to Harvard. Silver had been working on microbes that make hydrogen—with, she admits, limited success. So she was intrigued when Nocera approached her. Nocera founded the first artificial photosynthesis start-up, Sun Catalytix, in 2008. The company struggled, and, in 2013, it pivoted from working on a distributed solar-powered water-splitting system to a completely different sort of energy storage—flow batteries. In 2014, the company’s assets were acquired by Lockheed Martin.
Nocera wanted to find another use for molecular hydrogen: as a feedstuff for microbes. He and Silver turned to the bacterium Ralstonia eutropha, which can use hydrogen to fix CO2 and make polyhydroxyalkanoates, or PHAs, which are bioplastics.
The first system used cobalt phosphate to catalyze oxygen evolution and a nickel alloy to catalyze hydrogen production. In addition to PHA, Silver genetically engineered the bacteria to produce isopropyl alcohol, isobutyl alcohol, and isoamyl alcohol. In Silver and Nocera’s early work, nickel leaching in the reactor inhibited bacterial growth. So Nocera has replaced the nickel with a cobalt phosphorus catalyst.
UC Berkeley’s Yang has also developed a bionic system. Whereas Nocera’s reactor is electrocatalytic, Yang’s uses light directly. Semiconductor nanowires absorb light; catalysts coated on the wires split water to make hydrogen or electrons. Yang’s group has shown that bacteria can live comfortably on these wires and take up electrons or hydrogen from them, depending on the setup, to fix CO2. He’s also used multiple bacterial species working in concert to turn an initial product such as acetate into chemicals that are more useful to humans, such as natural gas and butanol. He says his next generation of the system will be simpler—he’s working on bacteria that can synthesize their own light-absorbing semiconductors.
Advertisement
So can microbes speed the progress of artificial photosynthesis to market? Yang doesn’t think so. “I consider this technology still at an early stage,” he says. “I’m not in a rush to scale up.” Yang, who is a chemist, favors the fully inorganic systems, which he is also working on. “It’s a better solution, a more durable one,” he says.
Silver disagrees. She notes that there are already large-scale industrial processes that rely on biofermentation. But she’s more interested in small-scale, distributed chemical production. Silver envisions the bionic reactor will be used in individual homes, perhaps to make hydrogen to power fuel cells or to make fertilizer. In that case, the system would be redesigned to fix nitrogen rather than CO2.
Nocera thinks artificial photosynthesis can’t work in developed countries at all—at least not in today’s economy. “We should have a long-term vision,” he says. “Someday, I think we should replace the petrochemical industry.” That won’t be anytime soon, despite the fact that “in the last 10 or 15 years, the number of scientific discoveries has been breathtaking,” he says. In the developed world, “you have a massive infrastructure you’ve already paid off,” and it’s built around petrochemicals.
He believes the bionic leaf is best suited for distributed chemical and fuel production in developing countries and places without centralized infrastructure. He says he’s working with Indian investors to license the technology he’s developed with Silver, but he can’t give any details yet.
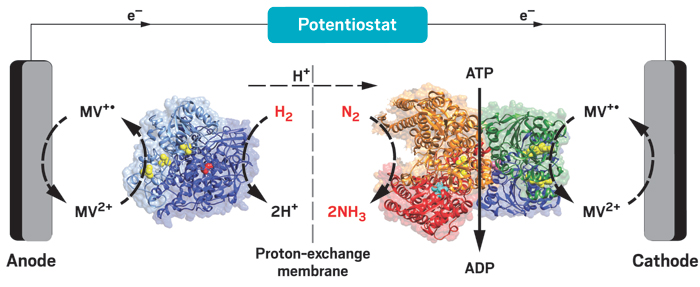
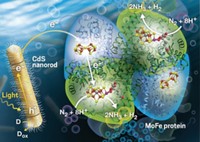
Turning sunlight into fuel
Various experimental systems use different methods to convert sunlight into fuels and other useful chemicals
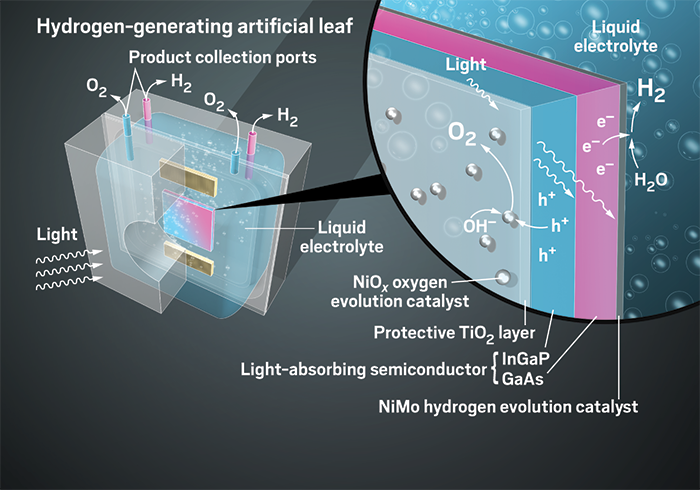
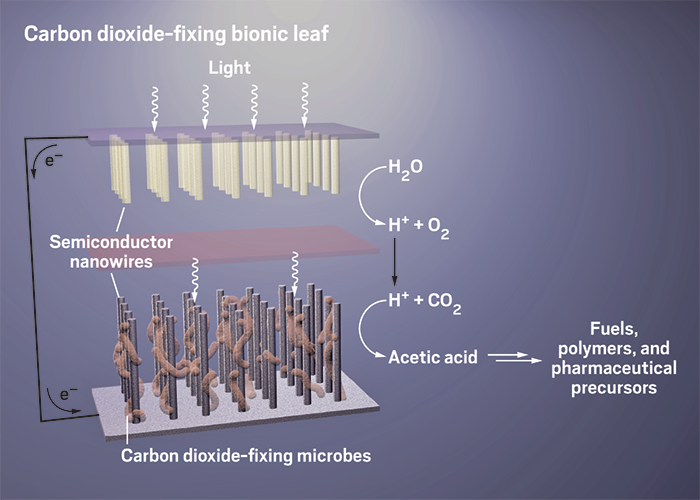
The lessons of leaves
Princeton’s Carter is skeptical of anyone who says they’ve cracked the photosynthetic water-splitting problem—or the CO2 reduction problem—to a degree that’s ready for market. Her attitude about different approaches to these challenges, though, is a welcoming one. “We have no idea where the breakthrough is going to come from, so we should be funding a lot of ideas,” Carter says.
To make the most progress, says Edward Sargent, a nanotechnologist at the University of Toronto, artificial photosynthesis research must be an international and deeply interdisciplinary project. There’s still much to learn about the natural enzymes that carry out photosynthesis, for example, which means chemists who work on catalysis should talk more with structural biologists and botanists. “This is really deep science. It’s interdisciplinary and will take some time,” he says.
Progress will also take continued investment and supportive policy from governments. Lewis says that a carbon tax, with revenues used to fund research on high-risk, high-reward technology research, would help. But after the recent presidential election, renewable energy researchers are saying all bets are off. The day after the election, Donald Trump appointed a climate change skeptic to head his Environmental Protection Agency transition team.
“The policy arena for this type of technology took a turn for the worse,” after the election, says Roger Sathre, chief scientist at the Institute for Transformative Technologies in Berkeley, a group that does research on how technology can address environmental challenges that affect the poor. JCAP started its second year of a planned five-year phase on CO2 reduction in September. Funds for future years will depend on annual appropriations.
“The last eight years, the government has been putting a lot into this,” Yang says. “We don’t want to see something like what happened in the ’70s.”
Yang is referring to the up-and-down funding landscape for clean energy research on photovoltaics and related technologies. Arthur Nozik, who was working then at what would become the National Renewable Energy Laboratory in Boulder, Colo., remembers those times. He was one of several researchers inspired by Fujishima and Honda’s original water-splitting work in the 1970s.
At the time, the energy crisis drove interest in alternative fuels. But then the crisis passed and clean energy research was cut in the 1980s. Researchers such as Nozik remember being dismissed as “hippies” by visiting senators who disdained their work on photovoltaics and other renewable technologies. Things started to change at the turn of the century, when the urgency of climate change became clearer. Now, the solar cell—something that once seemed implausible at today’s scales—has made it, but only after decades of diligent R&D.
Even if U.S. government funding fades, other countries may keep investing—most notably, Germany. From 2010 to 2016, the German government invested $107 million in chemical processes for CO2 utilization, with an additional $54 million from industrial partners. There, electrocatalysis is seen as a way to store electricity from intermittent renewable energy sources on the grid.
But for the most part, researchers who want to close the carbon cycle are keeping their heads down and working, not wringing their hands over the vagaries of markets and government policy. “If we can achieve our technology goals in terms of performance and cost,” Jaramillo says, “we don’t have to convince anybody.”
CORRECTION: On Nov. 22, 2016, this story was updated to correct how long Emily Carter has worked on methanol-generating photoelectrocatalysts.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter