Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Teaching aryl Grignards new synthetic tricks
Classic organometallic reagent reacts with bench-stable oxaziridines to forge C–N and C–O bonds
by Bethany Halford
December 1, 2016
| A version of this story appeared in
Volume 94, Issue 48

A time-honored laboratory exercise for students of organic chemistry is to make a Grignard reagent from magnesium metal turnings and an organohalide so that they can then use the resulting nucleophile to forge a new C–C bond. Chemists led by Rice University’s László Kürti and Brigham Young University’s Daniel H. Ess now report a way to use stalwart aryl Grignard reagents to fashion C–N and C–O bonds.
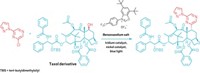
The chemists found that when they reacted aryl Grignard reagents with a bench-stable, bulky N-H oxaziridine, the nucleophiles attacked the compound’s nitrogen group to generate anilines. Blocking the oxaziridine’s nitrogen with a benzyl group prompted the Grignard to attack the compound’s oxygen atom instead, generating a phenol compound (Nat. Chem. 2016, DOI: 10.1038/nchem.2672).
“Our method is really plain and simple,” Kürti says. “You take either of these oxaziridine reagents and now you have the freedom to take the same aryl Grignard and make the aniline or the phenol out of it.” The reaction, he points out, uses simple reagents, low temperatures, and requires no special equipment, catalysts, or ligands. Kürti and Ess’s team used the transformation with more than 100 different aryl Grignard reagents, including ones that contain various functional groups.
“This is a nice new contribution to the current limited repertoire of useful electrophilic oxygen and nitrogen atom reagents capable of delivering these important atoms without unwanted functional groups,” says Jon T. Njardarson, an organic chemistry professor at the University of Arizona, who specializes in developing new reactions. “Given the importance of aniline- and phenol-type structures in pharmaceutical motifs, this new reaction is likely to make immediate impact.”
Kürti tells C&EN that he hopes a chemical supplier will start selling the oxaziridines so chemists can just buy them instead of synthesizing them. To that end, his lab is making the first 100 g for a well-known reagent company.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter