Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Turning blood cells into light-controlled drug carriers
Researchers store drugs in red blood cells as vitamin B12 conjugates and release them with light
by Stu Borman
December 1, 2016
| A version of this story appeared in
Volume 94, Issue 48

Red blood cells, the most numerous type of cell in the body, can act as light-activated drug delivery vehicles, according to a new study. The technique traps drugs in the cells, where they could circulate for the cells’ four-month lifetimes. Red or infrared light shone on a patient’s skin or through an inserted fiber optic could release the trapped drugs when and where they are needed.
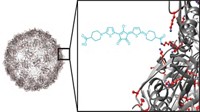
In the technique, David S. Lawrence of the University of North Carolina, Chapel Hill, and coworkers first covalently attach a drug and a fluorescent molecule to cobalamin, the core structure of vitamin B-12 (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201609731). The conjugates enter red blood cells passively when the cells’ pores are expanded in a low-salt buffer. But they can’t leave when the cells are in normal buffer or in the blood.
Red or near-infrared light, which can pass through tissue, excites the fluorescent group. This energy is then transferred to cobalamin’s corrin macrocycle, initiating a photolytic reaction. The reaction cleaves the drug from the complex. Because the drug is membrane permeable, it can exit the red blood cell to become biologically active in the patient’s bloodstream. Conjugates with different fluorescent groups could allow selective release of multiple drugs at different wavelengths.
The technique has so far been tested only in isolated red blood cells. In a treatment situation, a doctor would obtain red blood cells from a patient, load them with drug conjugates, and then re-infuse the cells.
Vladimir R. Muzykantov of the University of Pennsylvania, an expert in red blood cell-based drug delivery, comments that “drug delivery by red blood cells has been studied before in animals and is currently being tested in patients. But the level of precision and versatility of the new approach for controlled release of agents from red blood cell carriers is fascinating.” The next steps, he says, would be to test the approach in animals and the clinic.
The UNC group has started to test the strategy to treat autoimmune diseases and cancer in animals. Lawrence also founded Iris Biomed, in Chapel Hill, N.C., to commercialize the technology.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter