Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Engineered cells control blood sugar
Cells programmed to sense glucose and release insulin prevent hyperglycemia in diabetic mice
by Michael Torrice
December 15, 2016
| A version of this story appeared in
Volume 94, Issue 49

A team of bioengineers has developed a possible alternative to daily insulin injections for people with type 1 diabetes. The researchers engineered human kidney cells to act like pancreatic β cells, namely to sense blood glucose levels and produce insulin accordingly (Science 2016, DOI: 10.1126/science.aaf4006). When implanted in mice with type 1 diabetes, the cells prevent high blood glucose levels, also known as hyperglycemia.
Scientists have been working on ways to restore β cells destroyed by the immune system in patients with type 1 diabetes. For example, doctors have tested therapies in which they transplant islet cells, which contain β cells, into patients. But these potential therapies suffer from the same problem, says Martin Fussenegger of the Swiss Federal Institute of Technology, Zurich: They involve cells the immune system will eventually destroy. “β cells are not very robust cells; that’s why we have diabetes,” he says.
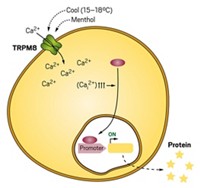
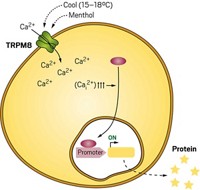
For that reason, Fussenegger and his colleagues decided to work with a heartier cell type, the human embryonic kidney (HEK) cell, and replicate the cellular circuitry that allows β cells to respond to blood glucose levels.
β cells regulate glucose levels with the help of three types of proteins (shown): glucose transporters, potassium channels, and voltage-gated calcium channels. Glucose enters β cells through the glucose transporters and is metabolized, producing adenosine triphosphate (ATP). As ATP levels increase, potassium channels shut down, stopping the outward flow of potassium ions from the cell. This changes the voltage across the cell membrane, which in turn activates voltage-gated calcium ion channels. Calcium ions then flow into the cell and turn on gene expression pathways that trigger insulin production.
HEK cells already have a glucose transporter and a potassium channel, so Fussenegger and his colleagues just had to engineer the cells to express the calcium channel and a calcium-dependent gene circuit for insulin production.
To test the resulting cells, the team encapsulated them in alginate beads and implanted them in mice that had their β cells destroyed chemically. The animals survived for the entire three-week experiment, while mice receiving nonengineered cells died after a few days, demonstrating that the β cell mimics could reverse fatal insulin deficiency. The engineered cells also restored normal glucose levels faster than implanted human islet cells.
John Pickup, a professor of diabetes and metabolism at King’s College London, says the new strategy is exciting, especially given its possible advantages over islet-cell-based therapies. Still, the kinetics of the insulin release from the engineered cells needs further tuning to better mimic that of β cells. The implanted cells, he says, don’t release the hormone fast enough to keep up with the typical blood glucose fluctuations a person experiences throughout the day.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter