Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microscopy
Chemists Nudge Molecule To React Then Watch Bonds Break And Form
Microscopy: Using STM and AFM, researchers trigger and visualize a chemical reaction at atomic level
by Stu Borman
January 26, 2016
| A version of this story appeared in
Volume 94, Issue 5

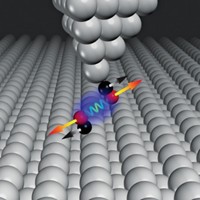
Researchers have used probe microscopy techniques to drive and then watch a chemical reaction proceed in both directions at a single-molecule level (Nat. Chem. 2016, DOI: 10.1038/nchem.2438). Leo Gross of IBM Research Zurich and coworkers there and at the University of Santiago de Compostela used scanning tunneling microscopy (STM) to push a molecule to react and atomic force microscopy (AFM) to image atomic-level details of that molecule as it formed radical intermediates and a final product.
The technique could allow chemists to initiate radical reactions by manipulating molecules at an atomic level, the researchers say. They note that the approach could be useful not only for making new chemical reactions possible but also for assembling functional molecules for molecular electronic devices and other applications.
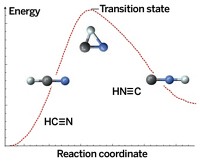
The team chose to study a version of the Bergman cyclization, a molecular rearrangement discovered by Robert G. Bergman of the University of California, Berkeley, in 1972, while at California Institute of Technology. In the reaction, an enediyne forms a diradical intermediate that then takes on two hydrogens to form a cyclized product. Some anticancer drugs, such as calicheamicin, work by cleaving DNA through the reaction.
In 2003, Felix R. Fischer and Michael F. Crommie of UC Berkeley used AFM to observe an enediyne cyclize by a similar reaction mechanism (Science 2013, DOI: 10.1126/science.1238187 and C&EN, June 3, 2013, page 7). In that work, the researchers heated the molecule to make the reaction occur.
In the new study, Gross and coworkers used voltage pulses from an STM probe to break first one and then another C–Br bond in the tricyclic compound 9,10-dibromoanthracene to form mono- and then diradical intermediates. The salt surface on which the researchers ran the reaction stabilized the radicals, allowing for AFM imaging. They then used another voltage pulse to convert the diradical to a bicyclic diyne.
The overall process is a ring-opening retro-Bergman reaction with an extra monoradical step that is not actually part of the Bergman mechanism. The researchers also demonstrated the reversible nature of the reaction by jolting the diyne to re-form the diradical intermediate.
The IBM study “is a real breakthrough,” says Wolfram Sander of Ruhr University Bochum, a chemist who studies reaction intermediates. The ability to visualize and push the system in both reaction directions “is a great achievement,” he says.
Peter Chen of the Swiss Federal Institute of Technology (ETH) Zurich, also a reactive intermediates expert, notes that the technique “allows the chemist to initiate the reaction of a single molecule and then see the bonding changes in that very same molecule—not quite directly, but as close to directly as one can possibly imagine. This corresponds to the state of the art of what can be achieved” with probe microscopy today.
This article has been translated into Spanish by Divulgame.org and can be found here.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter