Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Iron Catalysts For Enantioselective Hydrogenations
Catalysis: Abundant metal catalyst transforms ketones and imines into chiral alcohols and amines
by Bethany Halford
February 1, 2016
| A version of this story appeared in
Volume 94, Issue 5
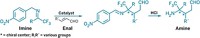
Making chiral alcohols and amines via enantioselective hydrogenation of ketones and imines is a cinch for chemists. But the ruthenium, iridium, and rhodium catalysts usually used for such transformations are expensive and toxic, which makes them unattractive for industrial applications. Because many pharmaceuticals, agrochemicals, flavors, and fragrances feature alcohols or amines with a stereogenic carbon at the α-position, chemists have been developing inexpensive, nontoxic iron catalysts capable of doing the same reaction. Until now, however, they haven’t managed to compete with the yields and enantioselectivity of the platinum-group metals. Antonio Mezzetti and Raphael Bigler of ETH Zurich report two iron catalysts (shown) that hydrogenate polar double bonds to produce chiral alcohols and amines with up to 99.7% yield and 99.4% enantioselectivity (Org. Process Res. Dev. 2016, DOI: 10.1021/acs.oprd.5b00391). This suggests that it’s possible to swap precious-metal catalysts for iron catalysts without sacrificing yield or enantioselectivity.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter