Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Fluorescence Method Maps Reactivity Hot Spots On A Catalyst’s Surface
Imaging: Technique could help improve catalyst performance and make reactions such as water splitting viable
by Mitch Jacoby
February 4, 2016
| A version of this story appeared in
Volume 94, Issue 6

Solid chunks of matter catalyze most of today’s industrial-scale chemical processes. If scientists knew exactly where reactions occur on these catalytic solids, they could customize them to improve the performance of catalysts already in use and help bring new ones to market.
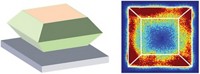
By devising a fluorescence microscopy technique with single-molecule resolution, researchers at Cornell University have created a method for pinpointing the sites of catalytic reactions with nanoscale resolution. The scientists used the method to generate surface maps of microscopic catalytic hot spots and then boosted the activity of those spots by decorating them with a cocatalyst (Nature 2016, DOI: 10.1038/nature16534).
The team, which was led by Cornell’s Justin B. Sambur and Peng Chen, applied the method to TiO2 nanorods used in light-induced water-splitting reactions. Solar-driven water splitting has been studied intensely for decades because it could provide a nearly limitless supply of clean-burning hydrogen if a suitable catalyst can be found.
When light shines on a TiO2 anode in a photoelectrochemical cell, short-lived excited electrons and positive charges form on the catalyst’s surface. The positive charges, called holes, can oxidize water, evolving O2 and forming hydrogen ions (H+). Then the electrons can reduce the ions to form H2. Often, however, electrons and holes quickly recombine, quenching the excitation before the catalytic reaction can occur.
Depositing a cocatalyst on the anode could help enhance the oxygen evolution step of water splitting. But knowing exactly where to put this oxygen evolution catalyst (OEC) is a challenge: Putting it in the wrong place can hinder light absorption, weakening the anode’s performance.
To find the answer, the Cornell team scanned nearly 40 TiO2 nanorods for catalytic hot spots by using two types of probe reactions—an electron-induced reduction and a hole-induced oxidation. Both reactions convert a nonfluorescent organic molecule that’s been added to the water-splitting cell to a fluorescent one. The molecule lights up immediately, identifying where on the nanorod it was converted. From those measurements, the team made catalytic activity maps and used them along with the microscopy method to selectively deposit tiny quantities of a cobalt borate OEC on the nanorod hot spots. This deposition improved the nanorods’ water-splitting abilities.
An imaging technique that uses cleverly chosen fluorescent reporter molecules to examine the fate of photogenerated electrons and holes in a semiconductor at the nanometer scale is “a breakthrough,” says Bruce A. Parkinson, a photocatalysis specialist at the University of Wyoming.
Not only did the Cornell scientists devise a sensitive mapping method, they made several unexpected observations. For example, most of the nanorods contain just a couple of reactive hot spots and large, relatively unreactive areas, even though the composition and structure of the rods are fairly uniform. Oxidations and reductions also tend to occur in nearly the same spots. And even though adding a cocatalyst to a hot spot makes it hotter as expected, adding the OEC to a “cold” spot on the TiO2 surface causes an improvement in catalytic activity that’s greater than the improvement at the hot spot.
Many of the insights obtained in this study are somewhat counterintuitive, Wyoming’s Parkinson says. Researchers working in this field will have to take them into account in future studies of photodriven catalytic reactions, he adds.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter