Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists Achieve Hydrocyanation Without Using Toxic Hydrogen Cyanide
Organic Synthesis: Safer, reversible transfer reaction to make nitriles opens up new possibilities in chemical synthesis
by Stephen K. Ritter
February 18, 2016
| A version of this story appeared in
Volume 94, Issue 8

Hydrocyanation is a go-to reaction for converting an alkene to a nitrile, which is one of the most versatile functional groups available to chemists. The process is used industrially to produce adiponitrile, an intermediate for making nylon. However, it is rarely used in the lab or for fine chemicals production because the hydrogen cyanide required as a reagent is extremely toxic, volatile, and potentially explosive.
Researchers in Germany have now designed an approach to hydrocyanation that not only avoids HCN’s pitfalls but is controllably reversible, which could make the reaction even more valuable (Science 2016, DOI: 10.1126/science.aae0427).
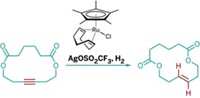
Xianjie Fang, Peng Yu, and Bill Morandi of the Max Planck Institute for Coal Research use a nickel catalyst as a shuttle to pluck hydrogen and a cyano group from a donor nitrile and transfer them to an alkene to form a nitrile. The team shows the reaction is useful to make aryl nitriles and for functionalizing biomolecules such as tyrosine and estrone.
In addition, the transfer hydrocyanation is made reversible on demand by selecting starting reagents that control the thermodynamic equilibrium of the reaction—the nitriles can be reverted to complementary alkenes. The team uses this retrohydrocyanation to make styrene, terpene, and aliphatic alkene derivatives from nitriles.
The reversibility provides two main advantages, Morandi says. It guides selective formation of linear alkyl nitriles, in contrast to normal hydrocyanation where branched products are obtained. And in retrohydrocyanation mode, the nitrile group acts as a removable activating group for the construction of C–C bonds.
“This practical and safe protocol for the HCN-free transfer hydrocyanation of alkenes is likely to fertilize nitrile chemistry,” notes organic chemist Hans-Günther Schmalz of the University of Cologne in a commentary accompanying the research paper. Schmalz envisions the approach stimulating new applications in academic and industrial labs, and perhaps in fine chemicals production. But he adds that the stoichiometric amounts of by-products formed could limit its use by companies already accustomed to safe handling of HCN.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter