Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Tiny enzyme tweak expands substrate palette
Changing just two amino acids transforms a picky aldolase into a cosmopolitan catalyst
by Mark Peplow
February 26, 2016

To be used industrially, enzymes usually need to undergo some surgery before they can become chemical workhorses. It may take a nip here, or a tuck there, to sculpt their active site into a shape that will accept a range of chemical substrates. But the alterations that allow an enzyme to be more promiscuous can also cripple its chemical activity or make it much less stable.
Researchers have now given a particularly picky enzyme a makeover so that it can handle a wide range of aldol reaction substrates without any loss in performance—all by changing just two amino acids in the enzyme’s active site (ACS Catal. 2016, DOI: 10.1021/acscatal.5b02805). “It was really surprising that it was so easy to do,” says Wolf-Dieter Fessner of the Technical University of Darmstadt, co-leader of the research team. The modified enzyme is “definitely a good candidate for industrial use.”
The aldol reaction forms a carbon-carbon bond between two carbonyl compounds—one acting as a nucleophile, the other an electrophile—to create a β-hydroxy carbonyl product.
Aldolases are generally involved in sugar metabolism, but engineered forms of the enzymes have been used in industry. For example, chemical manufacturer DSM modified 2-deoxyribose-5-phosphate aldolase (DERA) to accept chloroacetaldehyde as the aldol electrophile, and used it to synthesize the side chain of blockbuster cardiovascular drug Lipitor (atorvastatin) at industrial scale (Biotechnol. J. 2006, DOI: 10.1002/biot.200600020).
But adapting aldolases to accept a wider variety of nucleophiles, without losing their activity, has proved to be a much tougher challenge.
Along with Pere Clapés of the Institute of Advanced Chemistry of Catalonia, Fessner and colleagues set out to redesign D-fructose-6-phosphate aldolase (FSA). The wild-type enzyme, found in Escherichia coli, prefers small three-carbon aldol nucleophiles like hydroxyacetone or dihydroxyacetone and rejects larger ones.
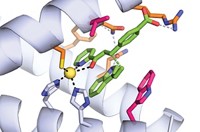
The team used the crystal structure of FSA to build a model of the substrate-binding pocket and identified key amino acid residues that could be altered to enlarge the active site. Then they designed modified DNA sequences for new enzymes, expressed a range of these mutants in the lab using E. coli, and tested them with a variety of aldol nucleophile substrates.
In the most successful mutant enzyme, two leucine residues deep in the active site were switched for alanines. This change expanded the pocket so that it could comfortably fit nucleophiles containing up to seven carbon atoms and including functional groups such as ethers and a terminal alkene. Reactions using 3-hydroxypropanal as the electrophile gave aldol products in yields ranging from 25 to 89%, with very high stereoselectivity. Remarkably, this mutant was almost as stable as the wild-type aldolase: On average, it denatured at 81°C compared with 87°C for the wild-type enzyme. “That was a surprise for me,” says Fessner.
Although small changes to a few amino acids can often have dramatic effects on an enzyme’s specificity, achieving that without sacrificing chemical efficiency or stability is a much rarer feat. “That’s what’s interesting here,” says Donald Hilvert, a protein engineer at the Swiss Federal Institute of Technology, Zurich (ETH). “It means that it’s potentially practically useful.”
The mutant enzyme was not perfect, though, proving unable to tackle nucleophiles with bulky substituents next to their carbonyl group. Fessner and his colleagues are continuing to shape the active site to make the enzyme’s tastes even more cosmopolitan.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter