Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
DNA offers new way to find antibodies and diagnose disease
Super-sensitive method harnesses PCR to detect antibodies in patient samples
by Erika Gebel Berg
March 8, 2016

Detecting antibodies as a way to diagnose disease is like trying to find one red ball among thousands of blue ones in a giant swimming pool. In patient samples, the antibodies of interest are few and far between—especially in saliva and urine, and early in the course of a disease. Now, researchers have developed a polymerase chain reaction (PCR) method for detecting antibodies that is 1,000 times as sensitive as enzyme-linked immunosorbent assay (ELISA), the gold standard test (ACS Cent. Sci. 2016, DOI: 10.1021/acscentsci.5b00340).
Infections, cancer, and autoimmune illnesses are often diagnosed with ELISA. In this method, a molecule known to bind the antibody—the antigen—sits on a plastic slide and captures antibodies present in a sample for detection. ELISA, though, has a couple of drawbacks, says Carolyn R. Bertozzi, a chemist at Stanford University and editor-in-chief of ACS Central Science. “Some antigen proteins unfold when bound to the ELISA plate,” she says, and an antibody won’t stick to the unfolded antigen. Radioimmunoassays get around this issue by detecting antibodies in solution, but they are labor intensive and involve potentially hazardous radioactivity. The other problem, Bertozzi says, is that ELISA isn’t usually sensitive enough to detect the low concentration of antibodies found in saliva or urine, or in blood during the early stages of a disease. Bertozzi’s team realized that PCR, being solution-based and highly sensitive, could solve both problems.
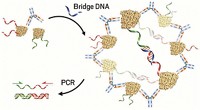
As a demonstration, the researchers attempted to detect antibodies associated with autoimmune thyroid disease. People with this condition produce antibodies that bind to thyroglobulin, a protein made in the thyroid. Thyroglobulin unravels on ELISA chips, making radioimmunoassay necessary for diagnosis. To harness PCR for the detection of thyroglobulin antibodies, the researchers first attached single strands of DNA to thyroglobulin. They used two different DNA sequences, creating two types of DNA-conjugated thyroglobulin. The researchers then incubated the DNA-antigen conjugates with a blood sample containing thyroglobulin antibodies.
Each thyroglobulin antibody can bind two molecules of thyroglobulin, bringing the tethered DNA strands in close proximity to each other. The researchers also added to the mix a piece of bridging DNA. When a thyroglobulin antibody bound one of each type of DNA-tethered thyroglobulin, the bridging DNA linked the two strands, creating a long piece of DNA with a double-stranded section.
“The only reason this works is because the antibody is holding the DNA strands close together in space, allowing them to be ligated,” says Bertozzi. Next, the researchers ran the PCR reaction to amplify any DNA with the target sequence. The final step was to look for amplified DNA using a fluorescent molecule that binds to the double-stranded DNA, plus gel electrophoresis to confirm it’s of the correct length.
The researchers successfully amplified the diagnostic DNA sequence from patient blood serum samples, demonstrating the presence of the antibody, while no signal was detected in a sample from healthy controls. Their approach detected 100-100,000 antibodies in 2 µL of serum, says Bertozzi, which is 1,000 times as sensitive as ELISA.
Mark W. Pandori of the University of California, San Francisco, says this approach appears to be sensitive enough to detect antibodies in saliva. “In the public health world, we want to test a lot of people in a resource-poor area, where it’s not easy to do blood draws. In those situations, you’d want to test oral fluid,” he says. “If we could test saliva as easily as blood, that would be a big advancement.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter