Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
A nanowire battery that won't die
Gel electrolyte dramatically extends nanowire electrode lifetime to 100,000 charges
by Katherine Bourzac
May 2, 2016
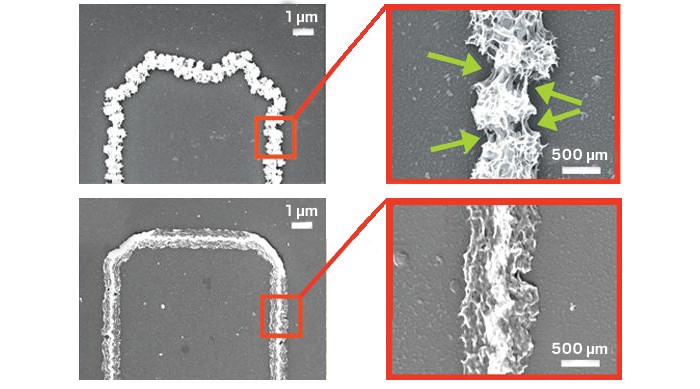
Imagine a battery that doesn’t wear out before the mobile phone it powers—one that never stops holding charge. An experimental energy-storage electrode that combines nanowires with a gel electrolyte could fit that bill. It lasts as long as the researchers have had the patience to charge and recharge it—so far, more than 100,000 times (ACS Energy Lett. 2016, 10.1021/acsenergylett.6b00029).
Nanowire battery electrodes can provide a lot of power in a small footprint, says Reginald Penner, a chemist at the University of California, Irvine, which should make smaller batteries possible. In a lithium-ion battery, the key to high power is enabling the lithium ions to flow quickly in and out of the cathode. Nanowires have tremendous surface area relative to their volume, which makes fast flow possible. The catch, says Penner, is stability. “Nanowires are really prone to breaking if there’s any corrosion,” he says.
Penner’s group has developed a test system that pushes promising nanowire materials to extremes, and in their latest work they have used it to study manganese dioxide nanowires, a potential lithium-ion battery cathode material. The test device is made up of just 750 manganese-dioxide-coated gold nanowires: They use so few because if a small number break, they can immediately see the degradation in battery performance. And the wires are extremely long—about 5 millimeters. That gives them plenty of places to break, making the test system very rigorous.
Graduate student Mya Le Thai discovered this surprising improvement as she was testing the manganese dioxide nanowires. In her experiments she replaced the typical liquid electrolyte used in the lab with a polymethyl methacrylate (PMMA) gel electrolyte—commonly used in solid state batteries. While the nanowire cathodes paired with the usual liquid electrolyte survived 2,000 to 8,000 charge cycles, remarkably, the one with PMMA did not fail even after 100,000 full charges and discharges—and even though it was being tested in a system designed to encourage failure.
Penner says he doesn’t know why a change from a liquid to a gel electrolyte makes such a large difference, but the group has a few theories. It may simply be that the gel mechanically holds the manganese dioxide in place, preventing it from breaking off of the gold substrate
underneath. Beyond that, he says, “we think the gel slowly leaks into pores in the manganese dioxide and plasticizes it, preventing the coating from fracturing.” Since the ions travel through the electrolyte, this seepage doesn’t have any effect on the battery performance. But it sure seems to make it last longer, says Penner. The California group is now testing this plasticizing theory. They also plan to see whether gel electrolytes have this effect on other kinds of nanowires—and if other gels work even better.
“In principle you would never replace this battery,” says Penner, because you’d want a new gadget or cell phone long before the battery stopped holding a charge. Battery manufacturers are skeptical about the cycle life of nanomaterials, he adds. Penner hopes these results will change their minds.
Thomas Mallouk, a materials chemist at Pennsylvania State University who has made nanowire electrodes that lasted a more typical 10,000 charges, says it’s surprising that such a simple modification has such a huge effect on cycle life. He notes that the California researchers’ apparatus is a great test setup, but it doesn’t resemble a real battery or supercapacitor. However, he says, “there nothing that intuitively suggests this wouldn’t work” if tested in more realistic designs.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter