Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Chemical reaction lights the way for tracking microRNA in living organisms
Probes provide first-ever technique for visualizing small RNA sequences that control gene expression in vivo
by Alla Katsnelson
June 8, 2016

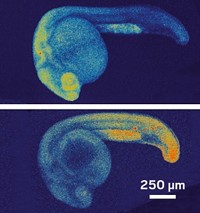
The ability to track molecular events inside the cells of living organisms offers a powerful window into fundamental biological processes, but methods for visualizing RNA in vivo without interfering with cell processes have been elusive. Now, researchers have developed a light-induced chemical reaction that accomplishes this feat in live zebrafish embryos (ACS Cent. Sci. 2016, DOI: 10.1021/acscentsci.6b00054). It is the first technique for detecting specific strings of nucleic acids in live vertebrates that doesn’t require genetically modifying the organism. What’s more, it’s sensitive enough to visualize the expression of microRNAs, small noncoding RNAs that act as puppetmasters of gene expression.
To do the reaction, chemical biologist Nicolas Winssinger, biochemist Marcos Gonzalez-Gaitan, and their colleagues at the University of Geneva designed two nucleic acid probes that each complement and bind to adjacent halves of a target microRNA sequence. The researchers conjugated one probe to a ruthenium complex that absorbs visible light and the other to a fluorogenic rhodamine that lights up when its azide bonds are cleaved. When the probes attach to the target sequence, the two reagents come close enough to react. Shining a light on the sample activates the ruthenium which then reduces the azide in the rhodamine conjugate, releasing its fluorescence. The dependence on external light allows researchers to control when the reporting reaction happens, Winssinger explains.
The team first developed the system three years ago (Chem. 2013, DOI: 10.1002/chem.201300060) for use in cultured cells; here, they adapted it for use in just-fertilized zebrafish embryos. “That’s really not trivial,” says Winssinger. The probes had to be nontoxic, stable for a day or more, and powerful enough to work even after being diluted through cell division.
After nailing down concentrations needed for an effective single-cell injection, testing reagents for toxicity and stability, and determining that the probes distribute homogenously throughout the embryo, the researchers designed three sets of probes against three different microRNAs, each expressed in a different type of tissue at various times: one expressed in neural cells during neuronal development, another in muscle cells as part of muscle differentiation, and a third in cells involved in forming the pectoral fins and tail. After the embryos were exposed to light for 30 minutes, confocal imaging revealed that each of the matched probes illuminated the expected type of tissue. The method also tracked how the gene expression changed over time in the neural cells as the embryo developed. The fluorescence maintained its intensity up to 36 hours post-fertilization.
Winssinger says the next step is to adapt the reaction for use in mice. And while visualizing microRNAs is one potential application for the reaction, he says, he also envisions using the same chemistry for other applications. For example, the same technique could provide a means of introducing bioactive molecules into the body that would be activated through the azide-reducing step of the reaction. “One could start to think about reprogramming some of the cellular circuitry,” he says.
The technique’s application in whole organisms is “a big step that is important for biology,” says Eric T. Kool, a chemical biologist at Stanford University. Applying it to organisms beyond the embryonic stage, however, would require a method for delivering the probes throughout the body of an animal. Still, many interesting experiments could be done with the technique in organisms under early development, he adds. “I think it’s already at the stage where it’s useful.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter