Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
ACS Meeting News: Radiochemical synthesis of fluorinated pyridine offers new view of the brain
by Steve Ritter
August 24, 2016

Developing new positron emission tomography (PET) tracers for biomedical imaging is one of the hottest areas of fluorine chemistry, and one of the most challenging. For example, using nucleophilic fluorination to develop meta-substituted pyridines that can be used as tracers is difficult because the aromatic ring is too electron-rich.
Chemical processes often need to be coaxed into action with heat. Catalysts can reduce the amount of heat required to drive a reaction, enabling an ordinarily high-temperature reaction to run at lower temperatures.
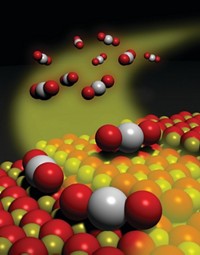
At the American Chemical Society national meeting in Philadelphia on Monday, Francisco Zaera of the University of California, Riverside, described an extreme low-temperature example: a gold catalyst that drives carbon monoxide oxidation at 150 °C below zero.
The finding broadens basic understanding of low-temperature chemistry and may lead to applications in space exploration.
Catalytic reactions that run at cryogenic temperatures are quite rare. One example is the esoteric interconversion of spin isomers of dihydrogen.
Reactions involving gold catalysts used to be rare. But in recent years, researchers have discovered several reactions mediated by nanosized gold particles. Those reactions, including CO oxidation, tend to run close to room temperature.
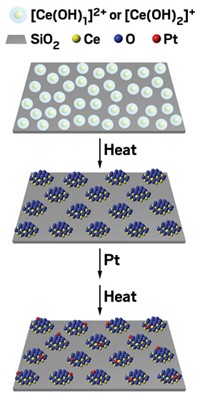
The UC Riverside group’s catalysts are also nanosized, but they do their job at a frigid 120 K. Speaking at a session sponsored by the Division of Catalysis Science & Technology, Zaera explained that his group prepares the catalysts by growing gold particles roughly 15 nm in diameter and encapsulating them in a 200-nm-wide silica sphere, which they in turn coat with a 20-nm thick titania shell.. The team then etches away the silica, leaving a yolk-shell structure—so named because it resembles an egg without the egg white (Surf. Sci. 2016, DOI: 10.1016/j.susc.2015.10.008).
The porous titania shell allows gases to diffuse to and from the catalytic cores and protects the gold particles from fusing together, a common problem that deactivates nanoparticle catalysts.
The amorphous titania shell contains sodium titanate and silicon impurities that prove critical to the cryogenic activity. Removing the impurities kills the catalysis. Spectroscopy measurements indicate that the mechanism governing cryogenic CO oxidation differs from the one active at room temperature.
Jeffrey G. Weissman, manager of catalyst development at Precision Combustion in North Haven, Conn, remarked that catalysts working at cryogenic temperatures could find applications in space exploration. For example, in an extended manned mission to an asteroid or Mars, astronauts may need to collect ice and other materials found on location and convert them to oxygen, water, fuel, and possibly plant growth medium. Weissman’s team has conducted research in support of such a mission and has developed cabin-air cleaning devices for the International Space Station.
“One aspect I find intriguing,” Weissman said, “is that the yolk-shell morphology provides a means to exclude certain gas species from reaching the catalyst.” For example, larger hydrocarbons or sulfur contained in a gas mixture may be physically blocked from reaching the catalyst surface thereby preventing catalyst deactivation. Such a capability could be important in devices that clean up exhaust gas or air in confined living spaces.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter