Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Lignin waste could sustainably safeguard titanium-based sunscreen
Coating titanium dioxide nanoparticles with lignin traps free radicals formed by UV light
by Deirdre Lockwood
October 7, 2016

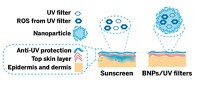
Titanium dioxide—a white pigment that reflects and scatters UV light—is a powerful sunblock. But one crystalline form of the pigment commonly used in sunscreens reacts in sunlight to produce free radicals that can damage skin. As a result, most sunscreen makers coat TiO2 particles with silica, alumina, or other compounds to quench their photocatalytic activity while preserving their sunblocking properties. A new study introduces a more sustainable alternative, encapsulating TiO2 particles with the abundant organic waste product lignin (ACS Omega 2016, DOI: 10.1021/acsomega.6b00177).
Lignins are a class of highly phenolic polymers that help give plants their physical structure. About 40 to 50 million tons of the waste is generated each year through production of paper, wood pulp, sugar cane, and other materials. But lignin is also a natural antioxidant—meaning it traps free radicals—and it absorbs UV radiation. These latter properties got Juan C. Scaiano and Anabel E. Lanterna of the University of Ottawa and their team thinking that it might serve as a sustainable, nontoxic coating for TiO2 in sunscreen.
To try it out, they mixed TiO2 nanoparticles with solutions of several different types of lignin, including water- and organic-soluble forms. They then irradiated them with UV-A, creating radicals that crosslink lignin, making it insoluble and causing it to adhere to the particle surface. After centrifuging and drying the product, the researchers recovered stable particles 30 to 50 nm in diameter, which included a 3- to 4-nm-thick lignin-based shell.
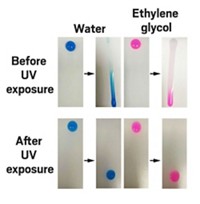
They first examined the coated particles using a standard test of photocatalytic activity, the oxidation of isopropanol to acetone under light exposure. When they exposed isopropanol solutions containing uncoated nanoparticles to UV-A and UV-B radiation, nearly all of the isopropanol was oxidized after three hours. In contrast, up to 90% of the isopropanol remained in solutions containing coated nanoparticles, suggesting the lignin had a protective effect against free radicals.
Because TiO2 has been shown to inactivate enzymes in the presence of UV light, presumably via free radical formation, the researchers also examined how the enzyme alkaline phosphatase behaved in the presence of the particles. In this case, the enzyme’s activity under exposure to UV-A light was reduced to about 20% of its initial value in the presence of the uncoated particles, but relatively unchanged with the coated ones.
Finally, to determine whether the coated particles retained their sunblocking properties, the team tested them with avobenzone. Though this common sunscreen ingredient protects against UV-A radiation, it rapidly degrades in sunlight, so it is often combined with other sunscreens to keep it stable over longer periods. In this case, the researchers made mixtures of avobenzone and either coated or uncoated particles and irradiated them with UV-A and UV-B. Coated particles protected avobenzone from photodegradation as well as or better than the uncoated particles did.
Paul Westerhoff, an environmental engineer at Arizona State University, says the lignin coating is a reasonable way to improve the properties of TiO2 and “potentially a good first step at using more natural materials,” but its performance should be compared with the coatings currently used in sunscreens, which include polydimethylsiloxane or aluminum silicates. He also suggests that the researchers investigate lignin’s stability—both its shelf life and its decomposition rates in the sun—as well as its hydrophobicity, since most sunscreens are combined with hydrophobic creams to make them spreadable and able to adhere to skin.
Two French cosmetics companies have expressed interest in the new approach, Lanterna says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter