Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Powering mass spec ionization with friction improves sensitivity
Charge generated by rubbing materials together ionizes tiny samples
by Celia Henry Arnaud
March 1, 2017
| A version of this story appeared in
Volume 95, Issue 10

If you’ve ever rubbed a balloon against your head to make your hair stand on end, you’ve experienced triboelectricity. Researchers at Georgia Tech are now putting such electricity—which is really just an electric charge generated by friction—to good use: They’re using triboelectricity to drive the ionization of molecules in a mass spectrometer.
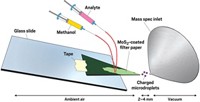
Materials scientist Zhong Lin Wang, mass spectrometrist Facundo M. Fernández, and coworkers replace the high-voltage power supplies that usually drive ionization with devices called triboelectric nanogenerators, or TENGs (Nat. Nanotechnol. 2017, DOI: 10.1038/nnano.2017.17)
These TENGs consist of a pair of electrodes and at least one pair of triboelectric layers, which are made of copper films on a polymer support. Movement of the layers relative to one another generates a charge that flows through the electrodes to an external circuit that includes a nanoelectrospray emitter. The layers’ surface area dictates the amount of charge generated. In the nanoelectrospray emitter, the charge ionizes molecules as droplets are generated from the sample solution.
The researchers made TENGs in two configurations. In one, the layers slide across each other. In the other, the layers press together and then separate. The sliding version generates charge pulses with alternating polarity, depending on the direction of motion. The pulses in both versions contain controlled amounts of charge.
With these devices, no external power supply is needed for ionization. “You can literally rub the two surfaces together by hand, and that’s enough to produce charges to ionize molecules,” Fernández says.
“Ionization methods in mass spectrometry are notoriously wasteful of the sample and produced ions,” says Akos Vertes, a chemistry professor at George Washington University. Conventional methods often produce ions that don’t make it into the mass analyzer.
Fernández and coworkers “have created a simple method for the on-demand generation of a predetermined amount of ions,” Vertes says. “The ability to control the amount of ions per pulse is an important advantage for efficient detection and analysis by a variety of mass spectrometers. This efficiency translates into superb sensitivity for a broad variety of samples.”
In fact, the TENG-powered nanoelectrospray source is more sensitive than a conventional nanoelectrospray source. For example, with the TENG, the researchers were able to detect 0.6 zeptomole (that’s 10−21 mol) of cocaine from a 10 pg/mL sample. A standard nanoelectrospray didn’t produce any detectable fragment ions from such a dilute sample. Fernández thinks the improved sensitivity results from a larger fraction of the charge going into generating ions and less charge being wasted than with a conventional power supply.
That sensitivity makes the source ideal for working with tiny sample volumes. “This is a great ion source if you have very precious samples,” he says. “You don’t want to consume the whole sample to perform your analysis.”
In addition to being small and sensitive, the source is also inexpensive. “It’s probably the cheapest ion source you can think of,” Fernández says. “You can literally build these for less than $2.00.”
Renato Zenobi, a chemistry professor at ETH Zurich who studies ionization methods, suggests that such devices could be used with portable or handheld mass spectrometers.
Fernández plans to use the source to analyze compound libraries generated as part of chemical evolution and origins-of-life research at Georgia Tech. He also hopes to revisit some of the work that his group has done previously to detect counterfeit pharmaceuticals.
This article has been translated into Spanish by Divulgame.org and can be found here.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter