Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Actinide complex covalency measured by EPR
Analysis of uranium and thorium complexes suggests further experimental data needed to benchmark computational results
by Jyllian Kemsley
January 9, 2017
| A version of this story appeared in
Volume 95, Issue 2
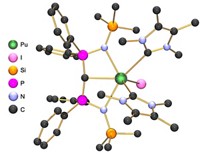
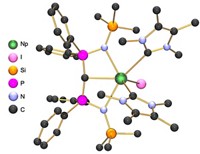
Mounting evidence suggests that actinide bonds have notable covalent character, making their chemistry more akin to transition metals than lanthanides. That covalency can be directly measured using pulsed electron paramagnetic resonance spectroscopy, yielding some surprising results, reports a team from the University of Manchester (Nat. Chem. 2016, DOI: 10.1038/nchem.2692). So-called superhyperfine coupling of a metal’s unpaired electrons with ligand nuclei that have non-zero spin can be used to assess covalency, but signals couldn’t be resolved by older EPR techniques. Using newer pulsed methods, Floriana Tuna, Eric J. L. McInnes, David P. Mills, and coworkers studied thorium(III) and uranium(III) organometallic complexes with ligands derived from the cyclopentadienyl anion (Cp). Computational analysis of the Th and U complexes suggested that they had very similar, weakly covalent bonds. But the EPR measurements indicate that the complexes differ, with much more covalency in U-Cp bonds than in Th-Cp bonds. Unexpectedly, the Th complex more closely resembles the analogous lanthanide ytterbium complex from the opposite end of the periodic table. “Such results highlight the need for new experimental data on systematic families of well-defined complexes,” the authors say.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter