Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Study confirms “hydrogen spillover” in catalytic hydrogenation
Dissociated hydrogens reduce substrate on two types of surfaces, but with drastically different efficiencies
by Stu Borman
January 5, 2017
| A version of this story appeared in
Volume 95, Issue 2

In some industrial processes, flowing hydrogen molecules over solid surfaces containing catalytic metals reduces organic molecules by hydrogenation. On the basis of a mechanism proposed in the 1960s, chemists think such reactions may proceed through the movement of hydrogen atoms in a process called “hydrogen spillover.” But researchers haven’t been able to confirm this mechanism because models of the reaction are difficult to create and analyze.
With a new, realistic model system, researchers have shown definitively that hydrogen spillover occurs on two key types of catalytic surfaces, called reducible and nonreducible supports, but to a drastically different extent. The information could help chemists design better catalysts as well as improve hydrogenation processes and hydrogen storage for fuel cells. The approach could also help scientists determine whether hydrogen spillover occurs more generally in important research and industrial reactions.
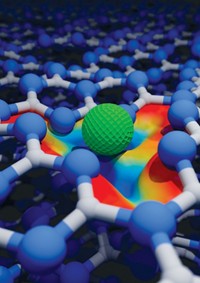
In hydrogen spillover, catalytic platinum nanoparticles on a solid support dissociate H2 into H atoms. The atoms migrate, or spill over, onto the support, where they can reduce a nearby substrate such as iron oxide. Chemists previously have shown that the process occurs readily on reducible surfaces such as titanium oxide but have struggled to observe it on nonreducible supports such as aluminum oxide.
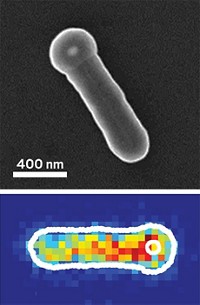
Yasin Ekinci of the Paul Scherrer Institute, Jeroen A. van Bokhoven of Paul Scherrer Institute and ETH Zurich, and coworkers used electron beam lithography to precisely deposit platinum and mixed iron oxide nanoparticles separated by various distances on TiO2 and Al2O3 supports. They then used X-ray absorption spectromicroscopy and density functional theory to analyze the extent to which H atoms reduced iron oxide.
Spillover is about ten orders of magnitude more efficient on TiO2 than on Al2O3, the researchers found (Nature 2017, DOI: 10.1038/nature20782). And on the nonreducible support, spillover is restricted to extremely short platinum-to-iron-oxide distances because of a competing hydrogen desorption process. The findings suggest that the potential of spillover-based hydrogenation is severely limited on nonreducible supports.
The enhanced understanding that the study provides could help improve the performance, lifetime, and costs of spillover-based hydrogenation systems, comments materials physicist A. Alec Talin of Sandia National Laboratories.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter