Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Electroplated batteries store more energy
A new process for making pure battery electrodes improves performance
by Katherine Bourzac
May 17, 2017
| A version of this story appeared in
Volume 95, Issue 21

Electroplated battery electrodes can store 30% more energy than today’s best commercial models, according to a new study. The electroplating process is compatible with a range of high-performance cathode materials called lithium transition-metal oxides. And it could help make flexible batteries needed for wearable electronics.
Making electrodes from these oxide materials normally requires high temperatures, which is a constraint on battery designs and performance, says Paul Braun, a materials scientist at the University of Illinois, Urbana-Champaign. The process starts by heating lithium and the transition metal of choice, such as cobalt, to 700–1000 °C to form an oxide powder. The high-temperature process ensures the oxide has good crystallinity, which is necessary for high performance. The resulting powder then gets blended with binders and other additives to make an electrode. These additives don’t store any energy, and they take up space and add weight, both of which are at a premium in portable electronics.

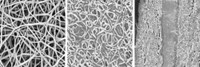
Braun’s group avoided using these space-wasting additives by electroplating the material directly on an electrode support. Because electroplating is driven by electricity, the required temperature can be lower than those usually needed to form the desired oxides.
The researchers placed foam electrodes or aluminum battery foils in a molten salt made of a mixture of lithium hydroxide, potassium hydroxide, and cobalt oxide. This made good quality films of lithium cobalt oxide on the foams and foils at 260 °C. Using this plating method, the group deposited three different cathode materials by switching out the cobalt oxide for manganese oxide and other compounds (Sci. Adv. 2017, DOI: 10.1126/sciadv.1602427). The process works well because this solution has a high ionic conductivity, and the transition-metal salts are highly soluble in it.
“It’s exciting to see a new idea” for making batteries, says Yi Cui, a materials scientist at Stanford University, who was not involved with the work. “They make lithium metal oxides with excellent crystallinity, and that shows up in the battery materials they’ve made.”
Braun says the electroplated materials have higher energy densities than those of state-of-the-art batteries because of the purity—around 90%—of the lithium cobalt oxide or lithium manganese oxide. With no additives, the electroplated cathodes can store more energy in a given volume. And the process is compatible with unconventional electrodes, including flexible mats of carbon nanotubes.
The question is whether electroplating will work at manufacturing scales, says Sehee Lee, a materials scientist at the University of Colorado, Boulder. Typically, battery electrodes are made on large-volume, roll-to-roll systems that run 24 hours a day. “If they can do this electroplating process on a roll-to-roll system that would be interesting,” he says.
Xerion Advanced Battery Corp. is now developing the electroplated cathodes, Braun says. He thinks they will be most useful in portable electronics, possibly flexible ones for future smart watch bands or apparel.
CORRECTION: On May 17, 2017, this story was updated to correct the scale bar in the micrograph.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter