Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Polymer ‘pulleys’ could boost Li-ion battery performance
Mechanical polymers prevent silicon anode disintegration
by Tien Nguyen
July 20, 2017
| A version of this story appeared in
Volume 95, Issue 30

Replacing the anode in commercial lithium-ion batteries with a silicon-based version could improve how much charge a battery can hold. But silicon anodes have been difficult to develop because they massively expand and contract as a battery charges and discharges, causing particles in the anode to break apart and the battery to fail.
Now, a team led by Ali Coskun and Jang Wook Choi at Korea Advanced Institute of Science & Technology has developed highly elastic polymers that, when added to a silicon anode, can relieve the stress of charging and discharging while holding the silicon particles together (Science 2017, DOI: 10.1126/science.aal4373).


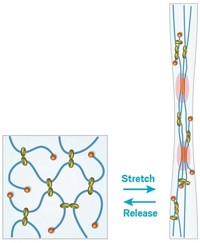
The researchers devised a unique polymeric network made of the conventional linear polymer polyacrylic acid covalently linked to polyrotaxanes containing mechanical bonds. In the polyrotaxanes, an amine functionalized polyethylene glycol chain is threaded through a number of cyclodextrin rings. During battery charging, as the silicon anode expands, the rings freely slide along the chain to dissipate stress, operating like a pulley system. The researchers showed that the molecular netting demonstrated excellent elasticity, withstanding up to about 400% strain.
The two main advantages of using this polymeric network are cost and processability, Coskun says. The team was able to make anodes with silicon microparticles, which are easier and cheaper to make in large quantities than the smaller and more commonly studied silicon nanoparticles, he says. Also, the new polymer accounts for only 10% by weight of the anode, compared with the 20% polymer binder typically used in silicon anodes.
The researchers are currently collaborating with a major battery maker to test their polymeric pulley system on real battery products.
“Mechanical bonds have come to the rescue for the first time in an energy-storage context,” says J. Fraser Stoddart of Northwestern University, who received the 2016 Nobel Prize for his work on rotaxanes and once advised Choi as a grad student and Coskun as a postdoc. The authors’ “ingenious” use of polyrotaxanes “marks a breakthrough in the performance of marketable lithium-ion batteries.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter